Active pharmaceutical ingredient is the term used to refer to the biologically active component of a drug product, such as a tablet or capsule.
Drug products are usually composed of several components and the active pharmaceutical ingredient is the primary ingredient. Other, inactive ingredients are commonly known as excipients and they are things like preservatives, dyes or flavoring. These inert substances are always required to be biologically safe. That doesn't mean they can't have an effect. An inert ingredient like lactose can cause an adverse reaction in some.
The procedure for optimizing and compositing this mixture of components used in the drug is known as formulation. For example, if the
active pharmaceutical ingredient
is a solid and the drug is required to have a liquid dosage form, such as a cough syrup, then the excipients would be the liquids that are used to formulate the syrup.
The design criteria for any small molecule
active pharmaceutical ingredient
is usually a combination of several factors that goes beyond the intended therapeutic effect, and usually heavily encompasses both pharmacokinetic and pharmacodynamic considerations, so for this reason, API molecules have many chemical functional groups.
The
active pharmaceutical ingredient
form that is used in a formulation is often the most thermodynamically stable crystalline form. As such, the phenomenon of hydrogen bonding in combination with there being many functional groups on the API usually results in the available crystalline form being a hydrate.
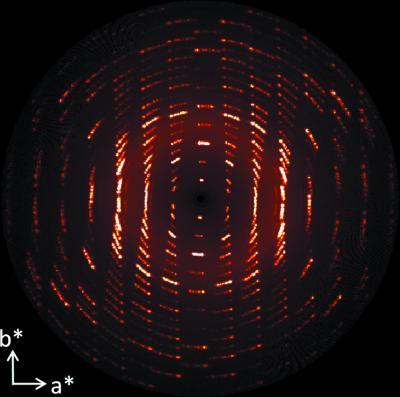
This image illustrates the observed reciprocal space reconstruction of hk0 section showing rotational smearing after heating the crystal. Credit: Chan et al.
Because of this fact the hydration behavior of crystalline APIs is of particularly high importance within the pharmaceutical industry, and is vastly studied from every possible angle. The state of hydration has a direct effect on the physical properties of the API, which in turn has a large impact on the drug processability and how the drug will eventually perform in-vivo, i.e. stability, solubility, and bioavailability.
Recently, a team of scientists in the department of Drug Product Science and Technology at Bristol-Myers Squibb developed a supplementary technique to complement more conventional analysis (methods such as calorimetric studies, nuclear magnetic resonance and vibrational spectroscopy) to study the behavior of hydration in organic crystalline solids.
By performing single crystal X-ray diffraction experiments with the ultimate objective being interpretation of the non-Bragg diffraction features, they were able to gain further insight into the mechanical and structural details of the dehydration of the crystal. These scattering features were reproduced and studied using computer models and the results were able to show the mechanistic relationships between changes in the lattice structure as stages of the overall drying process.
This study is the first of its kind to combine the mechanism of dehydration and non-Bragg scattering features from a single API crystal and the results will further improve the knowledge, formulation and choice of API used in drug manufacture today. The group at
Bristol-Myers Squibb
anticipates that these same data interpretation techniques will be useful to other researchers and that a more user friendly modeling software can be made available in the future.






Comments