Natural killer cells (NK cells) are part of our innate immune system. As they first line of defense, researchers agree that the body needs as many active NK cells as possible.
But, as is often the case, there can be too much of a good thing and researchers at the Helmholtz Centre for Infection Research (HZI) have shown how.
Usually, Listeria monocytogenes infection leads to a deadly sepsis in mice and immune suppressed humans. Interestingly, researchers observed that removal of NK cells during the early stages of Listeria monocytogenes infection improves survival of the mice.
Their new findings could help to prevent sepsis in the future.
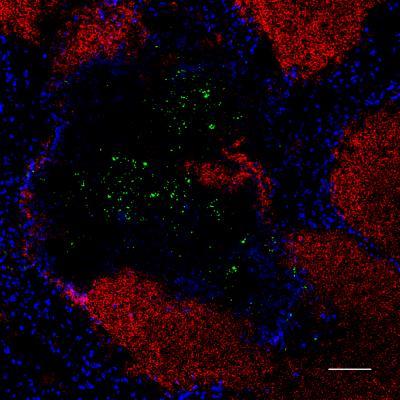
When infected with Listeria monocytogenes (green) it is important to have the right immune cells present at the right time. Too many natural killer cells for example can block the recruitment of neutrophilic granulocytes (red) which are vital for the immune response. Credit: © HZI / Jablonska
Until now it was believed that mice and humans die from listeriosis because their killer cells do not fight the infection effectively enough. However, the new results show the exact opposite. Even though the killer cells produce messenger substances that activate the immune system, the overproduction of the interferon IFN-γ causes problems. The large amounts of IFN-γ block the recruitment of neutrophilic granulocytes.
Those so-called scavenger cells are the most common white blood cells and are able to engulf bacteria and destroy them. If their migration to the site of infection is blocked in the initial phase, the bacteria can grow uncontrolled. This leads to fatal sepsis and death.
"During certain phases of the immune response it seems to be beneficial to have less active natural killer cells," says study co-author Dr. Jadwiga Jablonska-Koch at the Helmholtz Centre for Infection Research. "This is particularly true during the beginning stages of an infection, which is precisely when they were assumed to be most important".
However, having no active NK cells could also lead to uninhibited bacteria proliferation. In later stages of an infection NK cells are crucial for the survival of both; mice and humans. "It is important to find the right balance for NK cell activity. It has to be high enough to activate other immune cells, but at the same time the migration and activation of scavenger cells shouldn't be blocked," says Jablonska-Koch.
Where exactly this balance lies is still not clear. At least, the scientists were able to show that in the later phases of infectious diseases it can be beneficial for the body to have large amounts of highly active NK cells. The discovery of HZI scientists could be relevant in the future, especially from a medical point of view. If these results prove to be true for humans and also for other bacteria, they could help to develop new treatment strategies. "If we know how exactly the killer cells function, we could use this information for treatment of bacterial infections. But it is still a long way to go," says Jablonska-Koch.
Citation: Nuno Viegas, Lisa Andzinski, Ching-Fang Wu, Ronja-Melinda Komoll, Nelson Gekara, Kurt E. Dittmar, Siegfried Weiss and Jadwiga Jablonska, 'IFN-γ production by CD27+NK cells exacerbates Listeria monocytogenes infection in mice by inhibiting granulocyte mobilization', European Journal of Immunology 25 JUL 2013, DOI: 10.1002/eji.201242937






Comments