Nutrition and metabolism are closely linked with reproductive health. Several reproductive disorders including polycystic ovary syndrome, amenorrhea, and ovarian cancer have been linked to malnutrition, diabetes, and obesity. Furthermore, fasting in numerous species can result in decreased fertility, because the development of immature egg cells, called oocytes, is arrested. Understanding how nutrients accumulate in immature oocytes will provide valuable insights into the link between metabolic disease and reproductive dysfunction.
New work from Carnegie's Allan Spradling and Matthew Sieber focuses on the accumulation of triglyceride and a certain kind of steroids called sterols during oocyte development. They were able to identify an insect steroid hormone that is crucial to both lipid metabolism and egg production in fruit flies. Their findings are published in Current Biology.
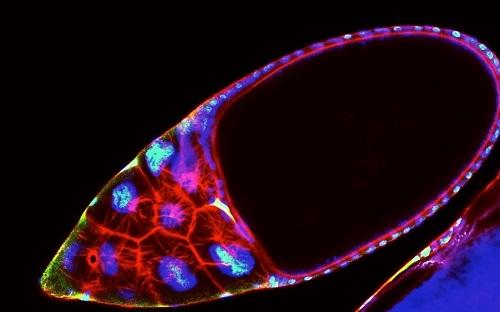
Ovarian follicle, the cytoskeleton is stained in red and the DNA in blue. The green shows the activation of SREBP. Credit: courtesy of Matthew Sieber
Recent studies in flies, mice, livestock, and humans have shown that lipids accumulate dramatically during the development of immature oocytes. These lipids appear to be required for healthy egg development and for early embryonic growth. But until now little was known about the mechanisms controlling this lipid accumulation in any species.
Spradling and Sieber discovered that the insect steroid hormone ecdysone stimulates a protein called SREBP, which controls the activation of genes that induce accumulation of lipids in immature oocytes. The lipids are important stored nutrients for the maturation of the oocytes and their development after fertilization. The SREBP control system has been highly conserved during evolution and controls lipid metabolism in humans as well.
They found that ecdysone also promotes a female-specific accumulation of high levels of stored fat and sugars throughout the body that is required for normal fertility.
Overall ecdysone acts to regulate systemic metabolism of the female fruit fly in order to support egg cell production. Without this female increase in stored body fat and sugar, oocyte development is slowed significantly fewer eggs are produced.
"Many metabolic mechanisms are conserved between fruit flies and humans, making the fly an excellent tool for defining the metabolic demands of reproduction in animals, including humans, and the regulatory pathways that fulfill these demands," Spradling said.






Comments