Enrollment began on March 17th, 2020. To be eligible, people had be enrolled within 3 days after confirmed exposure. 821 asymptomatic participants, 719 who had reported a high-risk exposure to a confirmed Covid-19 contact, were enrolled.
Participants were randomly assigned in a 1:1 ratio to receive either hydroxychloroquine (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg daily for 4 additional days) or placebo. Then they were monitored for laboratory-confirmed Covid-19 or illness compatible with Covid-19 for 14 days.
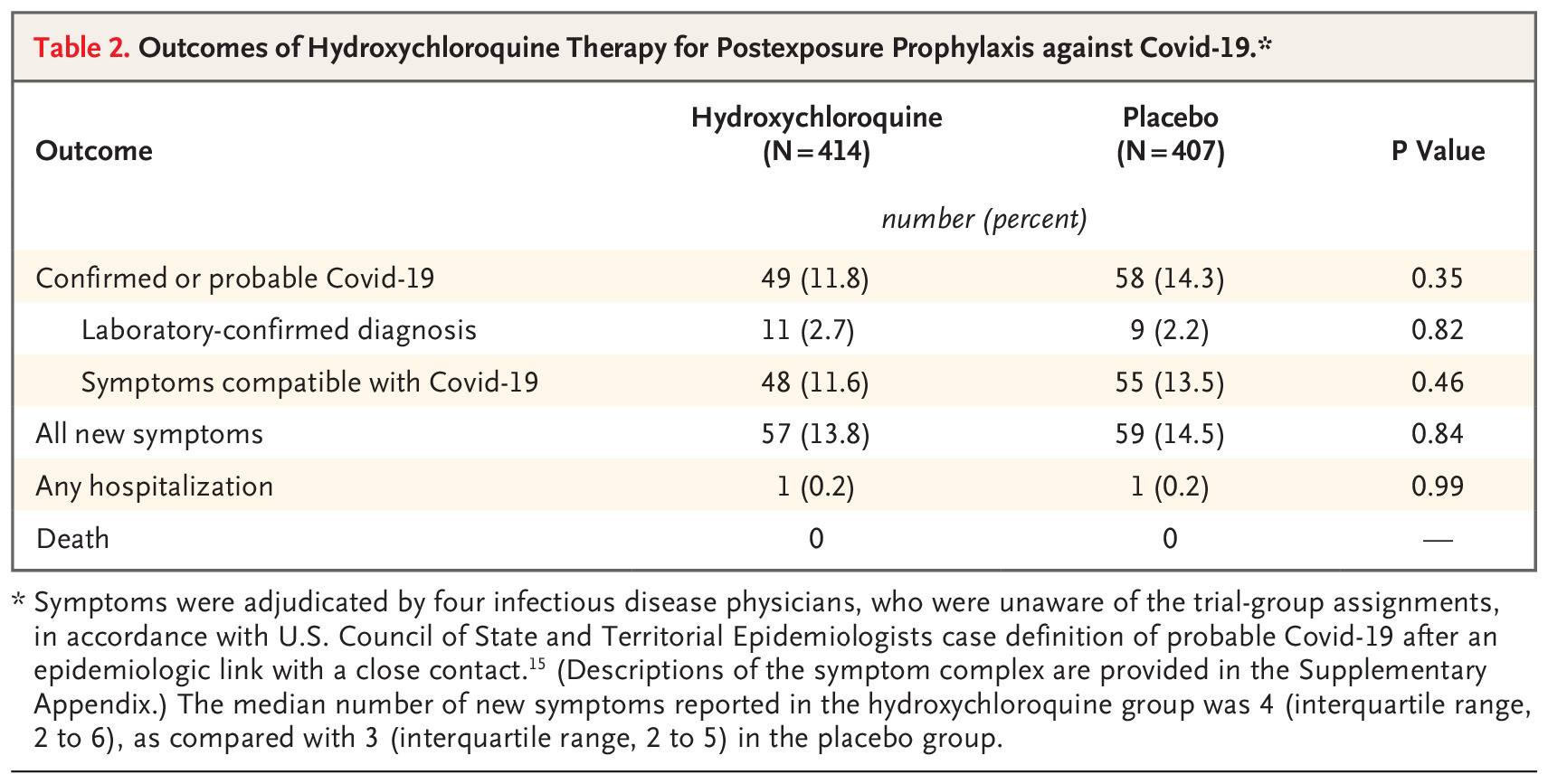
The incidence of new illness between participants receiving hydroxychloroquine and placebo was negligible. Side effects were more common with hydroxychloroquine but no serious adverse reactions were reported.
Citation: David R. Boulware, M.D., M.P.H., Matthew F. Pullen, M.D., Ananta S. Bangdiwala, M.S., Katelyn A. Pastick, B.Sc., Sarah M. Lofgren, M.D., Elizabeth C. Okafor, B.Sc., Caleb P. Skipper, M.D., Alanna A. Nascene, B.A., Melanie R. Nicol, Pharm.D., Ph.D., Mahsa Abassi, D.O., M.P.H., Nicole W. Engen, M.S., Matthew P. Cheng, M.D., Derek LaBar, Pharm.D., Sylvain A. Lother, M.D., Lauren J. MacKenzie, M.D., M.P.H., Glen Drobot, M.D., Nicole Marten, R.N., Ryan Zarychanski, M.D., Lauren E. Kelly, Ph.D., Ilan S. Schwartz, M.D., Ph.D., Emily G. McDonald, M.D., Radha Rajasingham, M.D., Todd C. Lee, M.D., M.P.H., and Kathy H. Hullsiek, Ph.D., 'A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19', New England Journal of Medicine, June 3, 2020, DOI: 10.1056/NEJMoa2016638




Comments