The U.S. Food and Drug Administration (FDA) has granted clearance to Geron Corporation to test their drug GRNOPC1, in patients with spinal cord injury. This allows the first ever human embryonic stem cell therapy clinical trial in humans. Geron’s Phase 1 will evaluate the safety of GRNOPC1 in patients with a specific category of spinal injury. Their trial will test patients with complete, grade A subacute thoracic injuries. Grade A complete spinal cord injury is defined by the American Spinal Injury Association (ASIA) as a person with no motor or sensory function preserved in the trunk (thoracic) and the lower extremities (sacral region).
Stem cells could provide therapies for patients suffering from paralysis injuries. Mouse embryonic stem cells stained with a fluorescent green marker for embryonic germ cells (precursor sex cells).
Credit: Niels Geijsen, Massachusetts General Hospital/National Science Foundation
The brain sends messages to muscles via electrical and chemical signals that are carried by nerve cells in the body. Electrial signals are carried through the body (axon) of each cell and through to the next cell's axon. Myelin acts as an insulation to the nerve cells which act as wires carrying electric currents. Myelin is made by specialized cells called oligodendrocytes, which are neural support cells. Disruption, caused by injury, of oligodendrocytes and the myelin surrounding the neurons halts the flow of electric signals and effectively stops communication, resulting in paralysis, motor and or sensory deprivation below the site of injury.
GRNOPC1 contains human embryonic stem cells (hESC) derived from oligodendrocytes, which are capable of facilitating remyelination and new nerve growth.Adding new myelin and growing new nerves in the injured areas can restore sensory and motor functions. This phenomenon has been documented in animal trials, where paralysis was partially reversed after treatment with GRNOPC1.
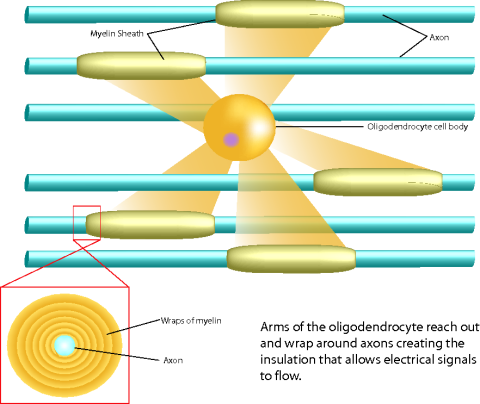
This illustration shows the relationship between axons, myelin sheaths and the oligodendrocyte cells in the spinal cord. Treatment with new stem cell derived therapies could promote repair of myelin and new oligodendrocyte activity in spinal injury areas. This could restore motor and sensory function in patients with spinal injuries. Photo credit: UC Irvine Reeve-Irvine Research Center
"Demyelination is central to the pathology of [spinal] injury, and its reversal by means of injecting oligodendrocyte progenitor cells would be revolutionary for the field,” said Richard Fessler, M.D., Ph.D., professor of neurological surgery at the Feinberg School of Medicine at Northwestern University. “If safe and effective, the therapy would provide a viable treatment option for thousands of patients who suffer severe spinal cord injuries each year."
The clinical trial is to be held in seven medical centers around the US, and will primarily evaluate the safety of the GRNOPC1 treatment. Efficacy will also be evaluated, as seen by improved neuromuscular control, and restored feeling. If seen safe, clinical trials will be extended to patients suffering from complete cervical injuries and also incomplete injuries, to enable treatment options for a broad spectrum of cases of spinal injury. According to Geron’s president and CEO Thomas Okarma, the “ultimate goal for the use of GRNOPC1 is to achieve restoration of spinal cord function by the injection of hESC-derived oligodendrocyte progenitor cells directly into the lesion site of the patient’s injured spinal cord.”
Although there are a lot of hurdles to still overcome, launching this clinical trial paves the way for new medical therapies for this and possible other previously untreatable conditions.





Comments