If you could live longer, would you be weaned on an extreme, emaciating diet?
The search for the foundation of youth has been happening forever and a popular idea in recent years has been caloric restriction - mice weaned on starvation diets live long and a new study of the tiny nematode worm C. elegans finds results even more alarming - it triggered a state of arrested development.
Though while the organism continues to wriggle about, foraging for food, its cells and organs are suspended in an ageless, quiescent state. When food becomes plentiful again, the worm develops as planned, but can live twice as long as normal. Over the last 80 years, researchers have put a menagerie of model organisms on a diet, and they've seen that nutrient deprivation can extend the lifespan of rats, mice, yeast, flies, spiders, fish, monkeys and worms anywhere from 30 percent to 200 percent longer than their free-fed counterparts.
Outside the laboratory and in the real world, organisms like C. elegans can experience bouts of feast or famine that no doubt affect their development and longevity. Ryan Baugh, an assistant professor of medicine at Duke University, found that hatching C. elegans eggs in a nutrient-free environment shut down their development completely. So he wanted to investigate whether restricting diet to the point of starvation later in life would have the same effect.
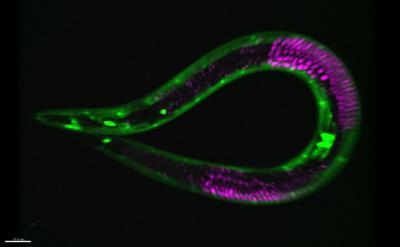
This shows the nematode worm C. elegans with muscle cells fluorescently labeled in green and germ cells fluorescently labeled in red. These cells and others pause at a checkpoint in development and slow their aging when worms encounter a period of starvation. Credit: David Sherwood Lab, Duke University
"It is possible that low-nutrient diets set off the same pathways in us to put our cells in a quiescent state," said David R. Sherwood, an associate professor of biology at Duke University. "The trick is to find a way to pharmacologically manipulate this process so that we can get the anti-aging benefits without the pain of diet restriction."
Sherwood and his postdoctoral fellow Adam Schindler decided to focus on the last two stages of C. elegans larval development -- known as L3 and L4 -- when critical tissues and organs like the vulva are still developing. During these stages, the worm vulva develops from a speck of three cells to a slightly larger ball of 22 cells. The researchers found that when they took away food at various times throughout L3 and L4, development paused when the vulva was either at the three-cell stage or the 22-cell stage, but not in between.
When they investigated further, the researchers found that not just the vulva, but all the tissues and cells in the organism seemed to get stuck at two main checkpoints. These checkpoints are like toll booths along the developmental interstate. If the organism has enough nutrients, its development can pass through to the next toll booth. If it doesn't have enough, it stays at the toll booth until it has built up the nutrients necessary to get it the rest of the way.
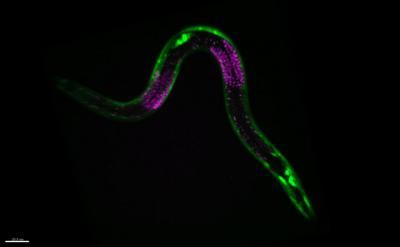
This shows the nematode worm C. elegans with muscle cells fluorescently labeled in green and germ cells fluorescently labeled in red. These cells and others pause at a checkpoint in development and slow their aging when worms encounter a period of starvation. Credit: David Sherwood Lab, Duke University
"Development isn't a continuous nonstop process," said Schindler, who is lead author of the study. "Organisms have to monitor their environment and decide whether or not it is amenable to their development. If it isn't, they stop, if it is, they go. Those checkpoints seem to exist to allow the animal to make that decision. And the decision has implications, because the resources either go to development or to survival."
The study found that C. elegans could be starved for at least two weeks and still develop normally once feeding resumed. Because the meter isn't running while the worm is in its arrested state, this starvation essentially doubles the two-week lifespan of the worm.
"This study has implications not only for aging, but also for cancer," said Sherwood. "One of the biggest mysteries in cancer is how cancer cells metastasize early and then lie dormant for years before reawakening. My guess is that the pathways in worms that are arresting these cells and waking them up again are going to be the same pathways that are in human cancer metastases."
The researchers are now performing a number of genetic studies to see if they can find another way to force C. elegans into these development holding patterns.





Comments