Neurotechnology may be on the verge of a major renaissance and mesh electronics could lead to a way to design personalized electronic treatment for just about anything related to the brain.
Scientists can detect the general areas of the brain where decision-making, learning, and emotions originate, but tracing behaviors to specific neurons is not yet possible. Right now, when the brain's complex circuitry starts to misbehave or degrade due to psychiatric illnesses like addiction or Obsessive-Compulsive Disorder, neurodegenerative diseases like Parkinson's or Alzheimer's, or even natural aging, patients have only two options for medical intervention: drugs or, when those fail, implanted electrodes.
Drugs like L-dopa can quiet the tremors that prevent someone with Parkinson's from performing simple tasks like dressing and eating. But because drugs affect more than just their target, even common L-dopa side effects can be severe, ranging from nausea to depression to abnormal heart rhythms. When drugs no longer work, FDA-approved electrodes can provide relief through Deep Brain Stimulation. Like a pace maker, a battery pack set beneath the clavicle sends automated electrical pulses to two brain implants. But each electrode is the size of a pencil.
During implantation, Parkinson's patients are awake, so surgeons can calibrate the electrical pulses. Dial the electricity up, and the tremors calm.
But, like with L-dopa, the large electrodes stimulate more than their intended targets, causing sometimes severe side effects like speech impediments. And, over time, the brain's immune system treats the stiff implants as foreign objects: Neural immune cells (glia cells) engulf the perceived invader, displacing or even killing neurons and reducing the device's ability to maintain treatment.
In contrast, mesh electronics provoke almost no immune response. With close, long-term proximity to the same neurons, the implants can collect robust data on how individual neurons communicate over time or, in the case of neurological disorders, fail to communicate. Eventually, such technology could track how specific neural subtypes talk, too, all of which could lead to a cleaner, more precise map of the brain's communication network.
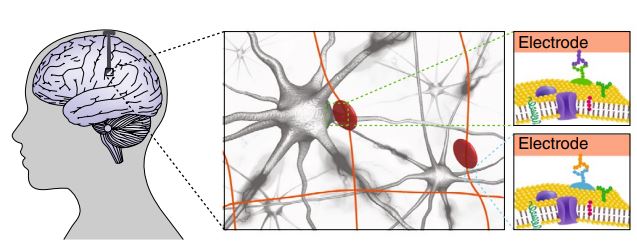
Schematic representation of syringe-injectable mesh electronic implant in a human brain. Credit: Nature
With higher resolution targets, future electrodes can act with greater precision, eliminating unwanted side effects. If that happens, they could be tuned to treat any neurological disorder. And, unlike current electrodes, mesh electronics have already demonstrated a valuable trick of their own: They encourage neural migration, potentially guiding newborn neurons to damaged areas, like pockets created by stroke.





Comments