Now that Philae has woken up, we may be on the brink of major steps forward in our understanding of comets. We already know that perhaps as much as 30% of a comet consists of dust and organics. Now we'll be able to look at this close up. Why, though, do most scientists expect Philae to find pre-biotic chemistry? Is there any chance of life? Also, where else in the solar system can we look?
So far, the only place we have searched is Mars, and our search there is in its early stages, focused on search for habitability. Recently we have started to plan spacecraft missions to oceans beneath the icy surfaces of Europa and Enceladus. What though about other ideas? Life in lakes of liquid methane on Saturn's moon Titan? Or in liquid nitrogen on Neptune's moon Triton? Sulfur based life on Jupiter's Io? Even lava flows of Venus have been suggested as a possible, though unlikely habitat. And what happened to Carl Sagan's idea of life in the atmosphere of gas giants? Stephen Hawkings championed this idea too. Is there any possibility of life there?
We have a good understanding of the geology of much of our solar system. However our biological exploration of the solar system has hardly begun. Philae will help to make some of our first steps in this direction with its direct search for life's precursors. This makes it the first "in situ" biological search since Viking.
WHY MOST SCIENTISTS EXPECT ONLY PREBIOTIC CHEMISTRY IN COMETS
Comets seem rather attractive places to look for life at first. After all, they have ice, and organics, and indeed, all the ingredients for life. Also short period comets come close to the sun regularly. For instance Comet 67P/Churyumov–Gerasimenko has an orbital period of 6.45 years. It wouldn't be that hard for microbes such as some of the ones from Antarctica to survive a six or seven year freeze.
However other things make it less likely that we'll find life on comets. The problem is, the comet is of course surrounded by vacuum. And in the vacuum of space, water can't stay stable. It just boils immediately into water vapour. To contain water as a liquid you'd need some impervious surface layer like the ice crusts of Europa and Enceladus.
So far, Comet 67p seems unaltered. It seems to be made up of many three meter diameter spherical "goose bumps" piled together, as you see in places where the interior is exposed to view
The roughly spherical bumps on the sides of this pit in the surface of Rosetta are all about 3 meters in diameter. They are remarkably consistent in size wherever they are spotted on Rosetta, suggesting that the entire comet interior may well be made up of these - which may be the "building blocks" that later came together to make comets.
This all points to a picture where the comets are undifferentiated primordial objects, loosely aggregated, and not likely to have liquid water habitats.
However, there are some puzzling observations which don't fit neatly into this picture.
Comet fragments don't survive impact into the Earth's atmosphere. They are too soft and mainly made of ice - so the only way to find out about them is to send our spacecraft to comets to study in situ or return samples. And that's what Stardust did, collecting fragments of dust from comet Wild as it flew past it.
Shows site of a particle impact into the stardust aerogel
Closeup of two of the particles detected in the Stardust aerogel, impact was from right to left.
The puzzle is, these samples returned from comet Wild shows clear evidence of alteration by liquid water in some of the smaller particles. The original paper in 2011 looks at various nickel, copper and zinc bearing iron sulfides in the comet and found evidence that they must have formed in low temperature hydrothermal environments (below 210°C). Several independent studies since then have confirmed this.
For a technical overview of the latest research see this paper under "Aqueous alteration in comets". I'll summarize what they say:
First, it's notable that they didn't find any of the clay like materials that are common in carbonaceous chrondite meteorites (polysilicates). It did have silica particles, so could have formed clays. But there were no clays at all, and all the particles collected larger than 2 microns were completely dry (anhydrous).
From this , it doesn't seem likely that the comet was altered by water - or at least - not the part that was the source of the dust collected in the flyby mission. If it was, it would have clays as well as the iron sulfides.
Comet debris in meteorite showers are typically similar in strength to soft snow. So that again suggests that it hasn't been altered by water. But if there were regions of the comet that were altered by water, they would be harder, like the carbonaceous chondrites, and it seems unlikely that they would be elected into space by the normal processes that produce the comet tails. They'd be too hard and dense. Impacts into comets would surely create liquid water temporarily, but it would just freeze solid and never escape into space.
So, the main question is - did this water found in the comet Wild particles form on the comet, or was it brought there from elsewhere in the solar system (incoming meteorites for instance, debris resulting from other objects in the Kuiper belt when it was in the outer solar system).
If this water formed on the comet, then it could be the result of debris from areas of the comet made liquid by impacts.
TEMPORARY WATER ON COMETS DOESN'T MEAN LIFE
If the water is the results of impacts, then these would only be the sites of liquid water temporarily and it seems unlikely that life would evolve there. We already have Carbonaceous chondrites, a rare type of meteorite that has organics in them, and show clear signs of alteration by water. These don't have any signs of life (at least not generally accepted), though lots of interesting organic chemistry. Perhaps their parent bodies just didn't have liquid water for long enough for life to evolve. So, temporary impact lakes on comets wouldn't seem to be long lived enough for life to evolve.
There's one dissenting voice here, however, Chandra Wickramasinghe. His model predicts liquid water habitats on comets that could be recurring and long term.
This theory is not widely accepted. The summary just mentioned says that his model seems "contrived". But let's have a look at it.
WICKRAMASINGHE'S THEORY
For a long time he and Fred Hoyle were just about the only ones arguing for panspermia with their idea that life originated in comets and spread to planets. Recently the idea of transfer of life between planets became mainstream, although through life spread in meteorites rather than comets.
His ideas are interesting then, though often controversial.
He has argued for many years that there may be liquid habitats inside comets where life may flourish even to this day.
His theory has two stages (from his 2009 paper)
First, he suggests that the comets originally had radioactively heated liquid cores in the early solar system, and this is when he thinks life may have originated. He would expect clays to form there, of course. But that's not ruled out by the Wild observations, as the clay would only occur in the interior of the comet.
As the radioactive elements decayed, then this source of heating was lost. But then short period comets could take advantage of the heating of the sun at perihelion. That's when the second stage of his theory comes into play.
CRUST OF "SUNBURNT ORGANICS"
In his model, the comet has a dark crust of "sun burnt" organics about 1 - 2 cm thick, which can frequently break and reseal. The solar heating heats the ice in a layer below this surface crust. The crust then traps the water vapour, at a saturation vapour pressure of 6 millibars. He predicts that these pools could form at depths from 2 cms downwards - and pools that form at depths of 30 to 50 centimeters below the surface could remain liquid throughout the perihelion passage of the comet. As the water vapour pressure increases some of the water would escape through cracks and pores in the organic layer forming the comet tails - but these would then reseal, so keeping most of the water liquid.
This model, if accepted, makes it possible to have liquid "puddles" of water just a short way below the surface of the comet which microbes could survive in.
Comet 67p does seem to have a hard crust, as we see from the "trampoline" effect, from the way that the Philae lander bounced off the surface - and this was a surprise to many who thought Philae would sink into a surface with the consistency of soft snow, or even less. I wonder if that is a point in favour of this model?

Of course you don't need to subscribe to his entire "life started in liquid core of ancient comets" theory to be interested in his model here. This hypothesis of liquid water beneath a surface crust of organics is logically independent of the hypothesis of a liquid core in the early solar system sustained by radioactive decay.
These recurring perihelion puddles, if they exist, could also be seeded by life from elsewhere (including Earth). Or they might not have life, but could have prebiotic chemistry altered in the presence of liquid water.
So - were the chemicals found in comet Wild the result of processes inside the comet, such as either a temporary impact lake, or these crust covered perihelion puddles? Or were they the result of delivery of material from elsewhere in the solar system?
And will we find any water altered chemicals on Comet 67p, and is there any chance that they could shed light on this question?
Whether or not we find anything out about that - there is one thing that surely we will surely get preliminary answers to, now that Philae has woken up.
EXCESS OF LEFT OR RIGHT HANDED MOLECULES
Organic molecules are often asymmetrical, and so, like our hands, come in two mirror image forms. Life uses only one of these. This is called its chirality.Philae is equipped with the first planetary exploration instrument designed with the capability to spot chiral imbalances.
Well - almost the first. Not the first ever, because Curiosity has a little known chirality experiment. It's part of SAM (Sample Analysis on Mars) . One of the traps for its gas chromatograph is a chiral compound, meaning that the rate at which compounds pass through it can depend on their chirality. On this page scroll down to GC4; trap; ChirasilDex (chiral compound separation). So in principle Curiosity also could perhaps do some chirality tests too.
But Philae's instrument COSAC, the Cometary Sampling and Composition experiment is the first ever dedicated "chirality module". It uses gas chromatography, like SAM, so gases percolate through a liquid column which separates out different molecules according to the speed with which they move - which then are analysed as separate molecules by a mass spectrometer.
The COSAC instrument used for chirality testing on Philae. The two large spheres here hold the carrier gas Helium. The samples are heated up in ovens, then the volatiles are carried into the experiment mixed in with the helium.
It has three chiral columns, Chirasil Dex CB, Chirasil L Val and Cyclodextrin G-TA. These have only one version of a chiral compound (only one enantiomer) instead of equal numbers of both. As a result, it can separate out molecules in the sample according to their chirality. These are then examined in detail by a mass spectrometer which examines each of the separated fractions independently. (Techy details of how it works).
An even more capable version of this technology will fly to Mars with ExoMars in 2020.
Some meteorites, like comets, are rich in organics, so these can be tested for chirality too. A famous example is the Murchison meteorite,
Fragment of the Murchison meteorite, and particles extracted from it in the test-tube. The meteorite was a witnessed fall, collected soon after it landed, and has many organics in it. It includes rare amino acids such as Isovaline:
Isovaline, a rare amino acid found in the Murchison meteorite. This helps confirm that the organics in it are of extraterrestrial origin as this amino acid is not involved in Earth life.
In this 2006 analysis the EET92042 and GRA95229 meteorites had chiral excesses ranging from 31.6 to 50.5%.
GRA95229 - another chrondite, collected in Antarctica, had chiral excesses of +31.6‰ for a-AIB to +50.5‰ for the (non terrestrial) amino acid isovaline, while the EET92042 meteorite ranged from +31.8‰ for glycine to +49.9‰ for L-alanine. It's thought that these excesses are extraterrestrial and not due to contamination by Earth life.
These are not thought to be signs of life in these meteorites, but results of prebiotic process. The idea is that Earth may have been seeded by organics with a chiral imbalance already.
So, in the case of meteorites, we have preliminary results already, and these chiral imbalances need to be investigated further with in situ study and a sample return mission.
We don't have any samples of these at all from comets yet though. Any that hit the Earth's atmosphere will just disperse completely. This will be our first chance to look close up at the ingredients of a comet.
This is a short video with chalk drawings introducing comets as a possible source for organics in the early solar system
Could comets explain why there’s life on Earth? A lesson in chalk
So it will be interesting to see if the organics in 67p have a chiral excess, and if they have it in the same direction as Earth life. Most scientists don't expect to find evidence of life there, but they wouldn't be surprised to find a chiral imbalance, such as we have already for some of the carbonaceous chondrites
MORE ABOUT PHILAE
If you want to find out more about philae and what it will do next, see Philae is awake! What's next for the comet lander's scientific mission?
OTHER PLACES IN THE SOLAR SYSTEM
So, now let's look further afield at some of the other places in the solar system that life could be found, or prebiotic chemistry.
LIFE IN LIQUID ("SUPERCRITICAL") CO2
We are used to CO2 as "dry ice" which turns instantly into a gas when it is heated. But, did you know that CO2 is liquid under high pressures? You can actually find liquid CO2 here on Earth too. At the high pressures of the ocean depths, anywhere below around 0.8 kilometers depth, CO2 is a liquid.
This video is taken at a depth of 1.6 kilometers at a white smoker vent system on one side of the small undersea volcano Eifuku off Japan
The "bubbles" in this video are in fact bubbles of liquid CO2. And there's life there, not actually living in the bubbles but apparently not bothered by it at all. See "Life in liquid carbon dioxide".
The first discovery of natural liquid CO2 in the oceans goes back to 1990.
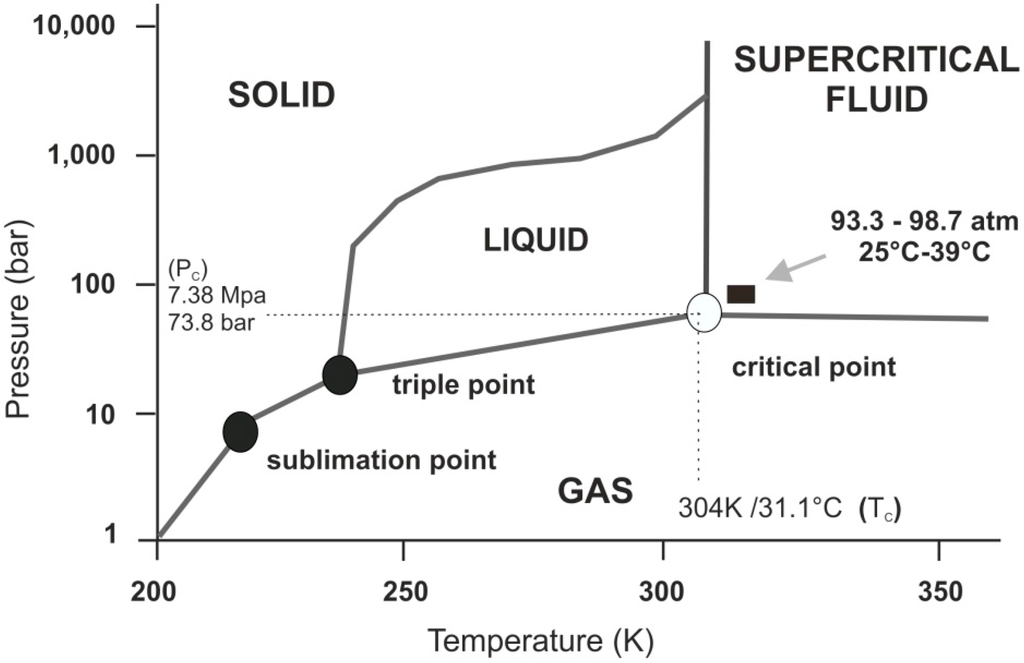
Interestingly, as you raise its temperature, liquid CO2 at around 31.1°C and 73.8 atmospheres in pressure becomes a supercritical fluid. That's a phase where the distinction between a gas and a liquid disappears. Often the properties change also.
Table from the paper Supercritical Carbon Dioxide and Its Potential as a Life-Sustaining Solvent in a Planetary Environment by Ned Budisa and Dirk Sculze Makuch
CO2 at these temperatures and pressures is actually a solvent like water. And indeed researchers Ned Budisa and Dirk Sculze Makuch found that it has advantages over water. Enzymes are more stable in liquid CO2 than they are in water, and it makes enzymes more specific about the molecules they bind to.
Supercritical liquid CO2 is often used for sterilizing. However, some microbes and their enzymes can tolerate living in it. This was a very surprising recent discovery reported in February of this year. The researchers found six strains of microbes, isolated from three sites targeted for geological carbon dioxide sequestration - that have the astonishing ability to grow on the interface between water and supercritical CO2. See Microbial growth under supercritical CO2.
There are fairly large reservoirs of liquid CO2 beneath the Earth's oceans. For instance a CO2 lake found off the coast of Taiwan at a depth of 1.4 kilometers.
At that depth, liquid CO2 is lighter than water, so it must be kept in place for instance by an overlying layer of clathrates (which they observed). They found life there, even in low numbers within the liquid CO2 layer itself. (Though in this case, the liquid is below the temperature at which CO2 is supercritical).
CO2 becomes heavier than water below a depth of around 3,000 km. There may be reservoirs below that level in the floor of our oceans, but they would be harder to detect, as no bubbles would escape from them.
You get supercritical CO2 on other planets too.
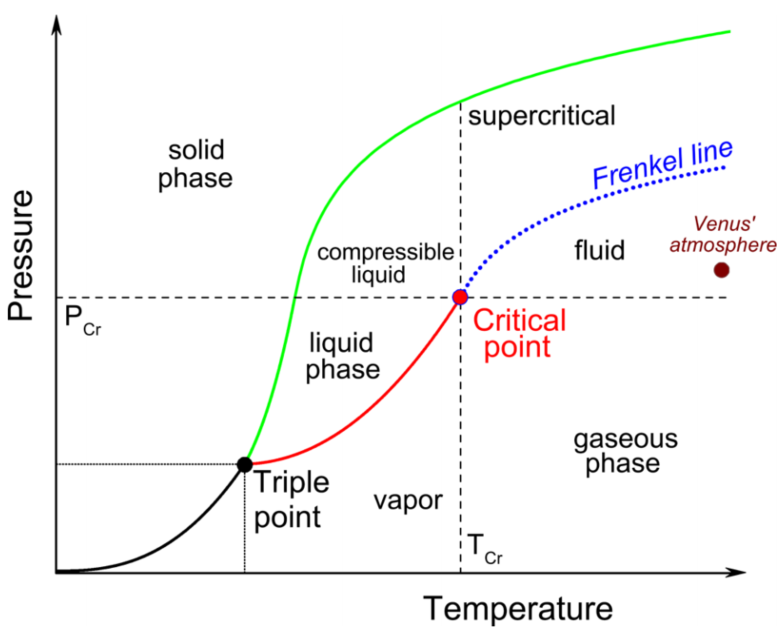
Indeed the whole of the Venus atmosphere is supercritical at ground level and for a short way above. However - it's not a likely place for life. It's too hot for organics to be stable. As well as that it's more of a gas than a liquid at those temperatures. That's because it is below the "Frenkel line" - the line above which a supercritical fluid has more liquid like than gas like properties.
Diagram from Structural Evolution of Supercritical CO2 across the Frenkel Line
However it may have a layer of supercritical liquid CO2 a short way below its surface, and in the past, when Venus's atmospheric pressure was probably several times higher (getting into the hundreds of bars), and the temperatures cooler, it might have had supercritical liquid CO2 oceans, which might have flowed like a fluid and carved out some of the features found on the surface.
You don't need a thick atmosphere or oceans for pressure though. Surface ice and rock layers would also lead to layers of liquid CO2, and this could happen on Mars.
Liquid CO2 would be stable on Mars beneath about 100 meters of rock in the cold conditions there. At one point this was the favoured explanation of the Mars gullies as many of them start at a depth of about 100 meters below the top of the cliff.
Combine those conditions with high enough temperatures, say from hydrothermal heating, and again you would have supercritical CO2 on Mars. Which might be a habitat for an exotic form of life.
LIFE IN ATMOSPHERES OF GAS GIANTS
This is an idea suggested by Carl Sagan in his Cosmos series.
For Carl Sagan's scientific paper on this, see Particles, Environments and Possible Ecologies in the Jovian Atmosphere.
And more recently by Stephen Hawking in his "Into the Universe"
In Cosmic Biology: How Life Could Evolve on Other Worlds, Louis Irwin and Dirk Schulze-Makuch look at this idea in some detail.
First they note that the upper layers with the visible clouds on Jupiter seem to be too cold for liquid water. And lower down, where the temperatures are warm enough, then there is little water. See page 166.
The main problem though, is how the life could evolve there in the first place. There are trace amounts of hydrocarbons, nitrogen compounds, and sulfur complexes. But there's a lack of oxygen, and there's no mechanism for concentrating the organics into one place. Any region where life could form is liable to be torn apart by the strong winds, and turbulence, and affected by strong radiation.
They thought there might be possibility of complex organics forming structures made up of large scale chemical networks of precursors and products. But without a hard and fast physical boundary or exact replication, then it wouldn't fit the criteria to count as life.
They thought it unlikely that Earth life could survive there, not being pre-adapted to the conditions. And indeed, Galileo at the end of its mission was sent to crash into Jupiter, for planetary protection reasons. For the same reason Cassini will be sent to crash into Saturn. The idea is that Earth life can't survive in either of the gas giant atmospheres, and by doing this we avoid the possibility of contaminating the interesting moons of these giants with Earth life.
Another idea is that the gas giant atmospheres could be seeded by life from their own planetary moons, such as Io for Jupiter, and Titan for Saturn. These may be similar enough in chemistry to the planets for their life to survive there. In case of Io, it would be pre-adapted to radiation and very low temperatures and to a sulfur based chemistry that could be found in the Jupiter atmosphere. And in the case of Titan it would be adapted to a dense, cold, organic atmosphere, again conditions that could be found in pockets in the Saturn atmosphere.
For details see page 168 of their book, most of which is available online.
SUPERCRITICAL LIQUID HYDROGEN LAYERS IN GAS GIANT ATMOSPHERES
Another possibility is life at a much lower level in the gas giant atmosphere where hydrogen becomes supercritical under high pressure and, like liquid CO2, it becomes a solvent for organics. On Jupiter this zone is very narrow but on Saturn then it is quite wide. This is suggested as a possibility in The Limits of Organic Life in Planetary Systems which was produced by the Space Studies Board.
LIFE IN LIQUID NITROGEN - PLUTO OR TRITON
This photograph of Neptune's largest moon Triton shows dark streaks thought to be nitrogen geysers.
Triton's south polar terrain photographed by the Voyager 2 spacecraft. The dark lines are the trails of plumes from volcanoes, thought to be caused by eruptions of liquid nitrogen from below the surface. Some of the plumes were actually observed erupting during the flyby- by anaglyph projection, which made them easy to pick out as they were closer to the spacecraft than the other features.
Triton - and perhaps Pluto also - could have thin layers of liquid nitrogen, between a surface of solid nitrogen ice and a subsurface of water ice. One researcher Jeff Kargel suggested that Pluto could have rivers of liquid nitrogen or neon. If not on the surface, it could flow below the surface since solid nitrogen is "a fantastic insulator".
So could there be life in these layers of liquid nitrogen?
Liquid nitrogen would be a non polar solvent (there is no separation of charge in its molecules), which means it can't dissolve organics (which are polar). But it might be just the thing for polysilanes, a complex molecule that uses silicon in the place of carbon. Maybe Triton and Pluto could have silicon based lifeforms?
It turns out that silanols - a kind of silicon version of alcohol - can dissolve in liquid nitrogen. And - in these conditions, silicon has as diverse a chemistry as carbon, as William Bains has argued in his hypothesis paper Many Chemistries Could Be Used To Build Living Systems.
The silanols have weaker bonds than carbon based organics - but this is just the thing you need in very cold conditions.
It's "silicon based life" but not in the sense that rocks are made of silicon - any more than humans are diamond or graphite or charcoal based life. The silicon of course is combined in long chains with many other atoms. See also Peter Ward's chapter on this idea.
LIFE IN THE OCEANS OF TITAN
Saturn's moon Titan is, so far, the only known place in the solar system with liquid water on its surface apart from Earth. Most of the lakes are around the north pole, with one lake, lake Ontario, at the south pole.
Glint of sunlight on the lake region around the northern pole of Titan.
Here is Chris McKay talking about prospects of life in the Titan lakes
He mentions there the provisional observations of hydrogen and acetylene and ethane depletion near the surface of Titan, which they'd predicted as a possible sign of life on Titan.
Also he mentions that oxygen would be in short supply, and need to be extracted from water ice "rock" by microbes. This is related, a possible way that life on Titan could make cell membranes without use of oxygen.
Titan is the only place in the outer solar system which we have sent a lander to, the Huyghens probe.
Sometime maybe we will send some more probes there to explore it further.
This was a recent idea for a submarine to explore Titan:
See also Life on Titan (wikipedia).
SULFUR BASED LIFE ON IO
There might be life on Io, sulfur based, in underground pools of liquid SO2, with the life chemistry probably also using H2S, because, though less abundant, it forms hydrogen bonds easily, and is more suitable for a solvent within the cells. So life might seek out the liquid H2S and use this as their intercellular solvent, while living in pools of the more abundant liquid SO2.
Volcanoes erupting on Jupiter's inner most moon Io, photographed by the Galileo spacecraft. The surface is rich in sulfur, and one suggestion is that Io could be host to an exotic sulfur based biochemistry.
Louis Irwin and Dirk Schulze-Makuch explore this in some detail in Cosmic Biology: How Life Could Evolve on Other Worlds,
The life molecules would have backbones of sulfur, nitrogen, phosphorus and other elements and would take advantage of the complex chemistry of sulfur compounds. Sulfur has a wide variety of oxidation states, even fractional oxidation states such as -0.4 or -2/3. It also forms a variety of ring compounds, and polymers.
The temperature range over which H2S and SO2 are both liquid is quite narrow, from -75°C to -60°C. And because these temperatures are quite low, the reaction rate is likely to be slower.
A plume ejected from same general region as one of the regions imaged by Voyager, called Masubi, it erupts 100 km into space from Io. The eruption comes from different places in this region and leaves plumes of SO2 on the surface.
Louis Irwin and Dirk Schulze-Makuch say in their concluding section of this chapter:
"It would be all underground and able to thrive only when temperatures reached an appropriate narrow range. But that would happen, for at least a brief period, in local pools or over short stretches of ground, every time a hot spot erupted or a sheet of lava advanced."
in Cosmic Biology: How Life Could Evolve on Other Worlds - Chapter 9, Fire and Ice (not available online).
WHAT ABOUT LIFE IN LAVA FLOWS ON VENUS?
One of the most challenging forms of life would be silicon based life living in liquid lava. If this was possible, we might find it on Venus - which has entire river channels that travel for huge distances across its surface.

The arrows show the two ends of this section of Baltis Vallis on Venus - the longest known channel of any kind in the solar system, total length of 7,000 kms. Though there is no liquid in it now, it may have been carved out by rivers of liquid lava, which, if not too hot, might have been a suitable place for life that relies on silicones.
These could use Silicones - organosilicon polymers with a silicon-oxygen backbone. These are stable at temperatures so high they would destroy any organics. But - would they remain stable at the temperatures of even cooling magma pools on Venus? That's the big question. Unlike water chemistry which is easy for us to explore in our laboratories - it's not so easy to explore the chemistry of silicones in magma pools.
Venus has no continental drift so doesn't have volcanoes all the time as Earth does - instead its entire surface may "turn over" every few hundred million years. But it shows signs of "young" features and may still be geologically active.
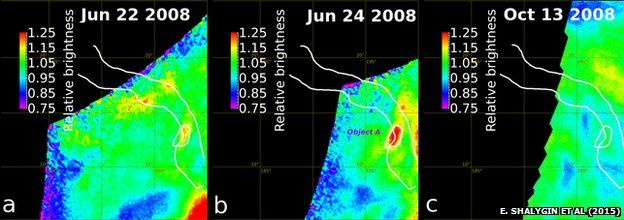
Recent observations show that some parts of the Venus surface may still be active today, with hot spots that appear and disappear, perhaps pools of lava, superheated rocks, or plumes of hot gas.
If life is possible in magma pools - then why hasn't it evolved on the Earth also? Why have we never found silicon based life fossils in lava flows on the Earth?
Silicon life seems unlikely to us. But is it just that it is a low probability life form? After all - for all we know organics based life, evolving in oceans, might also be low probability.
Maybe, just as we have no silicon based life in our lava, as far as we know - maybe, on other planets, there's no water based life in their water.
Maybe on other planets there are silicon based life forms reasoning in the same way that we do, that carbon based life is impossible.
Perhaps those silicon based life forms, if they exist, living in molten magma have lifeless seas of water, just as we have lifeless flows of lava (as far as we know anyway). To them, water might well seem too cold to sustain life which they think can only occur at the temperatures of molten lava :).
LIFE ON MARS
For this, I've written a lot about it already, so I won't say too much just now. In summary - back in 2008 few people thought that life was possible in present day Mars, except possibly deep below the surface. Now though, there are many suggestions for ways that life there could survive.
If you haven't read them, take a look at:
- Habitability Of Mars - Salty Seeps, Liquid Layers In Polar Ice, Ice Fumaroles, Sand Dune Bioreactors. ...
- Where To Search On Mars For Droplets,&Shallow Flows Of Liquid Water - Where Microbial Life May Flourish
- Rhythms From Martian Sands - What Did Our Viking Landers Find in 1976? Astonishingly, We Don't Know
LIVE IN THE OCEANS OF ENCELADUS AND EUROPA
Again I've written a bit about this here, so don't want to repeat myself to much, in short these are very promising places for life based on Earth like chemistry:
LIFE IN CLOUDS OF VENUS
The idea here is that Venus started off Earth like in the early solar system. But at some point it dried up, lost its ocean due to a runaway greenhouse effect, which didn't affect Earth in the same way because continental drift on Earth continually buries and circulates the carbonates.
Though the surface of Venus is amazingly inhospitable, the layer at the top of its clouds is in many cases the most habitable location in our solar system after Earth - almost Earth like in temperature, pressure, and atmospheric composition (without the oxygen of course). It has one major drawback, droplets of concentrated sulfuric acid.
However we do have acidophiles on Earth that survive in conditions not far off the acidity of Venus clouds - in sulfuric acid outflows from mines on Earth.
So - it's possible that there is sulfuric acid tolerant life in the Venus clouds. The other main problem with the high Venus clouds is that there are no solid surfaces of course. But it could have evolved in the early solar system and then migrated to the clouds as the surface got drier and hotter.
The main question is, could the life find some way to stay aloft? The residence time of particles is months rather than days - so - that makes it easier, and turbulence could return some of the life to the tops of the habitable layer after it reproduces - but it's still quite a challenge.
IT IS HARD TO JUDGE IF THESE EXOTIC FORMS OF LIFE ARE LIKELY OR UNLIKELY
You get people arguing that these forms of life are impossible because organic chemistry is so much more varied than any of these other types of chemistry. But how much of that is because organic chemistry is much easier for us to study?
We know a huge amount about how Carbon, Hydrogen, Oxygen and other atoms combine together at Earth pressures and temperatures, and in liquid water at laboratory temperatures. But is only rarely that experimenters try e.g. conditions resembling those of Titan, or Triton or Io. So it's perhaps not too surprising that we know many and much more complex compounds for organic based life.
Also, there is no way we could invent organic based life processes ourselves. We are only copying the way life does it and changing it slightly. And of course a lot of our organic chemistry is informed by and inspired by life chemistry.
So how much are we biased by our knowledge of how our own type of life works? And our natural tendencies to do experiments in conditions we find comfortable and using substances that are common where we live?
LOOKING FOR YOUR KEYS BENEATH A STREET LIGHT
It's a case, basically - of looking for your keys beneath the street lights first - because you haven't got a torch - and there is at least a chance that you might have dropped them there. It's quite a sensible thing to do.
“Did You Lose the Keys Here?” “No, But the Light Is Much Better Here”
If life is easy to find, and at least some of it is organic based - then perhaps we will find organic based life and maybe it won't take too long to find.
If it is rare, or most is not organic based, - then who knows how long it will take to find it? And who knows if we will recognize it easily when we first find it?
At the moment we can assume almost anything about life in the solar system.
We haven't sent life detection equipment to any of these places (except Viking to Mars in the 1970s). None of this life would be easy to spot from orbit, so there is no way to know at present.
It might be that life in one form or another is present almost everywhere in our solar system, even in the most exotic habitats. Or it may be present in some of them and not others. Or it could be present nowhere except on Earth.
If there is no life in these places, there may well be prebiotic or complex chemistry in some or many of them. These might hint at the range of possibilities for life elsewhere in the universe and also give insights into our understanding of the very early stages of life evolution.
FIND OUT MORE
I've only touched on a few highlights of a vast subject here.
For some more ideas, see
- The Limits of Organic Life in Planetary Systems (National Research Council)
- Hypothetical types of biochemistry (wikipedia)
- Cosmic Biology: How Life Could Evolve on Other Worlds, Louis Irwin and Dirk Schulze-Makuch
- Many Chemistries Could Be Used To Build Living Systems, William Bains
- Life as We Do Not Know It: The NASA Search for (and Synthesis of) Alien Life - Peter Ward
- Weird Life: The Search for Life That Is Very, Very Different from Our Own - David Tooney
Part of this started off as quora answers to: Why do scientist assume if there is no water or CO2 on alien planets that there is no life? and What planets/moons do scientists expect to find life in our solar system? I only know of Europa?
COMMENTS AND SUGGESTIONS
Do say if you have any ideas or suggestions or questions for that matter. Or share your own favourite exotic chemistries. And if you spot anything in this that needs to be corrected, however minor, including typos, be sure to say, thanks.
















Comments