Will your new year start with a BANG?
Well, it might, if you bring it in with this molecule. This is trinitramide, a compound newly synthesized at the Royal Institute of Technology (KTH) in Sweden. It has the formula N(NO2)3, and as such is the newest nitrogen oxide to be discovered.
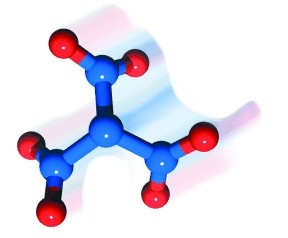
As a chemist, I am into stinks and bangs. And NO2 groups are already associated with explosives, from traditional ones such as nitroglycerine and TNT, modern ones like Semtex, through to even more powerful chemical specialities such as the highly energetic heptanitrocubane.
This compound first ‘appeared’ in quantum chemistry calculations, but has now been prepared. The question is, does it go with a bang or a fizz? The former looks, at first glance, much more likely.
But it might be more useful if it went with a fizz; then we might have the most powerful solid rocket propellant to date. As Tore Brinck, professor of physical chemistry at KTH, says:
“A rule of thumb is that for every ten-percent increase in efficiency for rocket fuel, the payload of the rocket can double. What’s more, the molecule consists only of nitrogen and oxygen, which would make the rocket fuel environmentally friendly. This is more than can be said of today’s solid rocket fuels, which entail the emission of the equivalent of 550 tons of concentrated hydrochloric acid for each launch of the space shuttle.”I can’t access the paper itself until after the New Year Bank Holiday (Jan 3rd), but I intend to watch this space.
References:
Paper abstract: http://onlinelibrary.wiley.com/doi/10.1002/anie.201007047/abstract
News item: http://www.nanowerk.com/news/newsid=19523.php





Comments