When the Chinese invented gunpowder round about the 800s, they founded one half of the science of chemistry, namely bangs, the other half of course being stinks.
They quickly applied it to warfare, both as an explosive in bombs, and as a propellant in rockets. It remained the explosive for about a millennium, but in the 19th century demands both from the military and from industry created a demand for new explosive.unpowder was a low explosive which burns swiftly rather than detonates.
As such it could propel a rocket, bullet or cannonball, but to create an explosion it had to be in a confined space such as a shell. Towards the end of the 19th century superior low explosives were made, which left less mess in the barrel than gunpowder and did not give off so much smoke which could betray a sniper’s or artilleryman’s position.
But the real development came in high explosives, which come in two main classes. Primary explosives are sensitive to shock or heat, and are used as detonators, while secondary explosives are much safer to handle in bulk, but need a small quantity of detonator to set them off. Mercury fulminate came on the scene in the 1830s as a primary explosive to replace flints in rifles for setting off t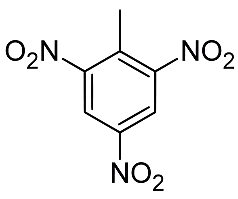 he main charge which was still gunpowder.
he main charge which was still gunpowder.
Among secondary explosives, Dynamite arrived in 1866 and was used for blasting, while TNT was invented in 1863 but took decades to be adopted for military use because it is relatively difficult to set off. What makes these explosives high is that all the components for the reaction are present in one molecule, so that in the TNT molecule (picture) nitro (NO2) groups correspond to the saltpetre in gunpowder, while the carbon part of the molecule corresponds to the charcoal.
Most explosives get their power from nitrogen. Nitrogen gas is a very stable molecule, but when nitrogen is combined with other elements, particularly oxygen, there is a large energy dividend to be gained by letting it re-form as nitrogen gas. With nitro groups, the liberated oxygen is ready for quick combustion with any organic matter that happens to be around.
These days, increasing demands are being made on both explosives and propellants, both in terms of performance and environmental impact. Standard propellants come in three main categories, liquid, hybrid, and solid. Liquid propellants consist of two ingredients, such as liquid oxygen in one tank and kerosene or liquid hydrogen (as in the Space Shuttle main rocket) in the other. More exotic recipes are also used, of which the one favoured by the Soviets was a mixture of unsymmetrical dim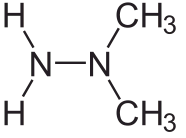 ethyl hydrazine (known as ‘heptyl’) whose formula is pictured here, and dinitrogen tetroxide.
ethyl hydrazine (known as ‘heptyl’) whose formula is pictured here, and dinitrogen tetroxide.
This can be used over a wide temperature range such as found in the extreme climates of the interior of the former Soviet Union, and is hypergolic, meaning that the two components ignite on contact and do not require an ignition system or detonator. But ‘heptyl’ propellant is notorious for having contaminated much of the former Soviet Union, especially near launch sites where primary booster stages were shed [1].
Liquid propellants are favoured for large rockets because with them it is much easier than with solids to control the burning. But they require sophisticated storage facilities, and for smaller rockets solid propellants come into their own, though I wouldn’t call the Space Shuttle Solid Rocket Boosters that sit in pairs on the sides of the main rocket exactly “small”. Here the main fuel is aluminium which is difficult to ignite, but once it starts gives a large amount of energy for a given volume (the converse of the fact that it requires a lot of electrical energy to extract it in the first place.) However, the oxidizer, ammonium perchlorate, reduces to hydrogen chloride gas, which is not nice, though I would say it is preferable to the nitrogen oxides given off by nitro-containing systems.
These days demands on explosives have also become yet more stringent, both in terms of performance and environmental impact. Detonators such as mercury fulminate and lead azide leave toxic heavy metal residues, while many nitro-type explosives give nitrogen oxides, and many kinds produce pollutant particles.
In regard to greening of rockets one approach taken at Stanford University is a hybrid system involving paraffin wax (giving the nickname “Candlestick Rockets”) and an oxidizing gas. For the latter, “flavour of the year” 2003 was oxygen, with the possibility of a cleanly burning mixture with minimal pollution. (group with rocket). Attention since seems to have moved to nitrous oxide or “laughing gas”.
The N20 molecule is in effect a nitrogen molecule with a loose oxygen atom on the end, and packs almost as much punch as oxygen itself, being used in the nitrous-oxide/acetylene flame to atomize elements for spectroscopic analysis. Unlike nitro-containing compounds, I guess that, since the two nitrogen atoms are already linked, this one would not leave the nasty “NOX” type nitrogen oxides that the catalytic converter in your car (aka “automobile”) is there to destroy.
For explosive AND propellants, a new class of materials called tetrazoles shows promise across the spectrum. These are tetrazoles, containing four nitrogen atoms in a five-membered ring, the other atom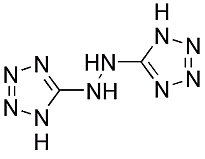 being carbon. Very active in the development of tetrazoles is the group of Thomas M. Klapötke at the Ludwig-Maximilian University of Munich, Germany. They are at present developing highly energetic bis-tetrazoles [2] such as HBT (right).
being carbon. Very active in the development of tetrazoles is the group of Thomas M. Klapötke at the Ludwig-Maximilian University of Munich, Germany. They are at present developing highly energetic bis-tetrazoles [2] such as HBT (right).
In the rings the nitrogen atoms are linked together by single and double bonds. Getting those four N atoms to re-arrange into two triply-bonded molecules pays a large energy dividend. There are many different tetrazole compounds, and small changes in chemistry can make the difference between a sensitive detonator and reasonably stable propellant. Moreover, the gases given off by these compounds, though not exclusively nitrogen, are much less toxic than those from many other explosives.
So we now have the promise to be environmentally friendly and easy-to-handle propellants.
When I was a lad (and the old folks were still going on about how much better mammoth steaks were fresh rather than frozen) it was still considered more or less obligatory for a chemist to be able to read German. This ability may have saved me from accident, because a 19th century paper warned me that something I was concocting could give rise to a compound which verpufft. But a title such as:
(Tetrazoles for the safe bang!) does make one think. If these are used in mining and road-building, there may well be less environmental impact, but if there is a demolition in progress, one should still keep well clear!
References:
http://www.chemie.uni-muenchen.de/ac/klapoetke/content/news/in_the_news_files/rocket.jpg
http://msnbcmedia3.msn.com/j/msnbc/Components/Photo_StoryLevel/080527/080526-friendly-bomb-02.hmedium.jpg
[1] A preliminary assessment of the potential environmental and human health impact of unsymmetrical dimethylhydrazine as a result of space activities
Lars Carlsen, Olga A. Kenesova, Svetlana E. Batyrbekova
Chemosphere, Volume 67, Issue 6, April 2007, Pages 1108-1116
[2] Bistetrazoles: Nitrogen-Rich, High-Performing, Insensitive Energetic Compounds
Thomas M. Klapötke and Carles Miró Sabaté
Chem. Mater. 2008, 20, 3629–3637





Comments