Doctors can now understand better chronic myeloid leukaemia (CML), including how it responds to therapy, thanks to a new mathematical model for the disease developed by scientists in Portugal, Belgium and the United States. The work, to be published in the June edition of the journal Haematologica, also reveals that current therapies – which are not believed to cure CML – with the right protocol can actually get rid of the disease, and provides guidelines on how to do that. CML although rare, because of effective life-extending therapies, is now one of the most common leukaemias in the world
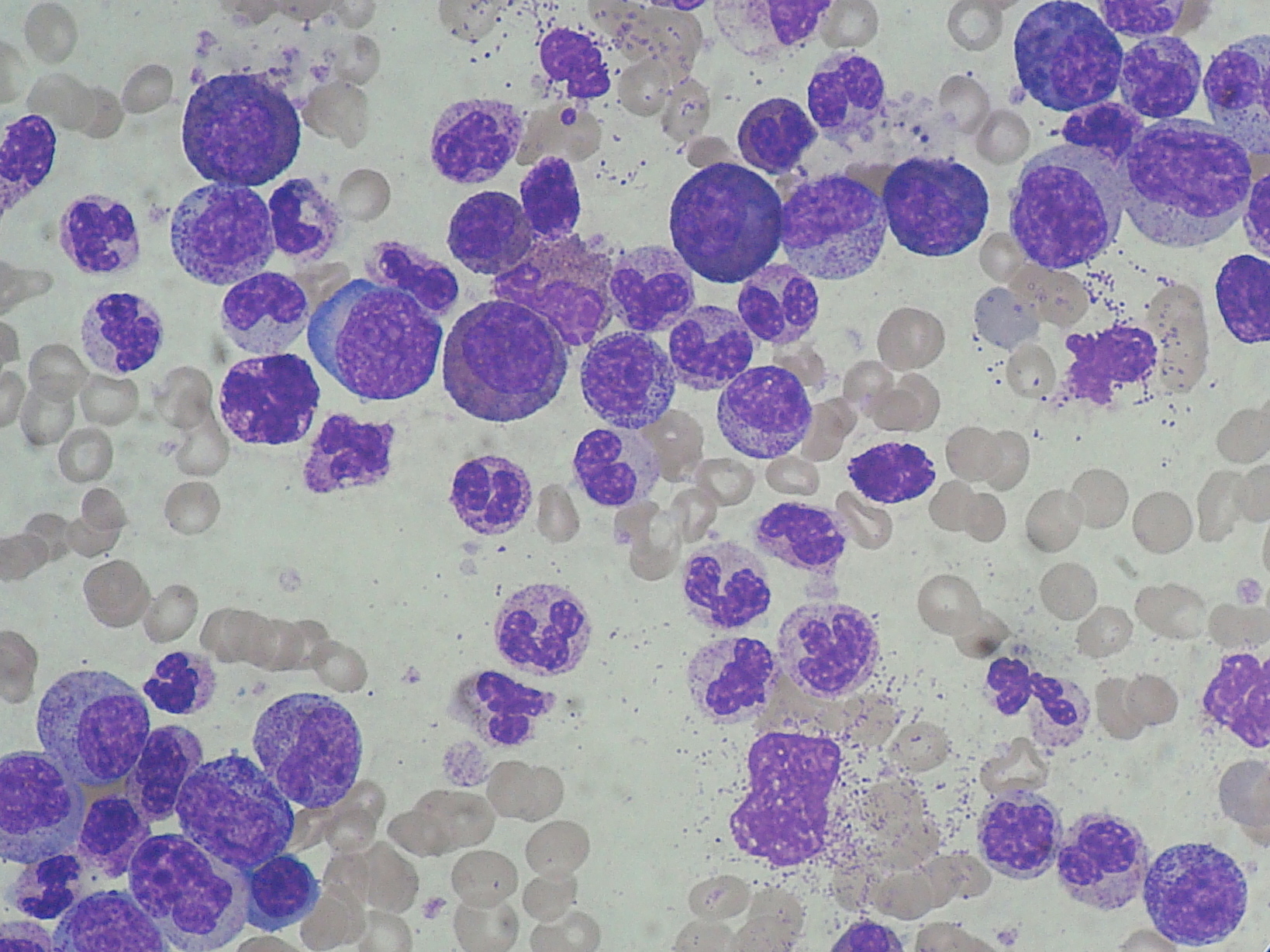
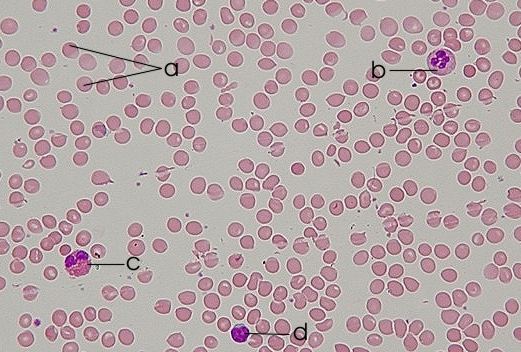 CML is a cancer of blood stem cells in the bone marrow (BM) that leads to the abnormal increase of granulocytes (a type of white blood cells that originates from these stem cells) disturbing the patient’ immune response. The mutated stem cells have a chromosome anomaly that forms a new gene called BCR-ABL which is then passed to the granulocytes and their progenitors (intermediate maturation state cells between stem cells and granulocytes) that develop from the affected stem cells. And while BCR-ABL seems to make no difference to leukemic stem cells (LSC), affected progenitors multiply without control as result of BCR-ABL capacity to activate many molecules linked to cell division. And while normally cells divide to form daughter cells that differentiate (maturate) to specific roles in the body, BCRL-ABL positive cells, instead, are much more likely to continue dividing. <?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
CML is a cancer of blood stem cells in the bone marrow (BM) that leads to the abnormal increase of granulocytes (a type of white blood cells that originates from these stem cells) disturbing the patient’ immune response. The mutated stem cells have a chromosome anomaly that forms a new gene called BCR-ABL which is then passed to the granulocytes and their progenitors (intermediate maturation state cells between stem cells and granulocytes) that develop from the affected stem cells. And while BCR-ABL seems to make no difference to leukemic stem cells (LSC), affected progenitors multiply without control as result of BCR-ABL capacity to activate many molecules linked to cell division. And while normally cells divide to form daughter cells that differentiate (maturate) to specific roles in the body, BCRL-ABL positive cells, instead, are much more likely to continue dividing. <?xml:namespace prefix = o ns = "urn:schemas-microsoft-com:office:office" />
Imatinib is a recent and very effective medication against CML that acts by blocking BCR–ABL, stopping the leukemic cells division and pushing them into maturating. This frees the BM space necessary for the remaining immune system to develop properly helping to restore the patient health (patients become immuno-compromised when the disease is in full bloom). But because the drug does not eliminate the LSC, from which new leukemic granulocytes and progenitors can still develop, it is seen as only palliative – very effective prolonging patients’ life but not curing them.
This is where the new work by Tom Lenaerts at the Free University of Brussels, Jorge M. Pacheco at <?xml:namespace prefix = st1 ns = "urn:schemas-microsoft-com:office:smarttags" />Minho University, Portugal, Arne Traulsen from the Max-Planck-Institute for Evolutionary Biology, Germany and David Dingli at the Mayo Clinic College of Medicine in the USA enters. They claim that all the studies of CML so far have ignored the effect of random factors, which will be amplified (and so impossible to ignore) in populations as small as those of stem cells. They then develop a new computer model that takes into account these processes and simulate the evolution of CML in a million of virtual patients with surprisingly results.
In fact, their new model predicts that 5 years after the first leukemic stem cell appears around 84% of the patients should have lost them already, suggesting that CML is driven, not by LSC, but by progenitors. What is more, after only 1 year about 50% of the patients are predicted to be LSC-free.
But how can CML be diagnosed if there are no LSC?
The reason –according to the researchers- lies in the fact that not only leukemic immature cells tend to multiply very slowly, but also - because of the “BCR-ABL effect” – they get “stuck” dividing in each maturation stage (from stem cells to mature granulocytes there are about 32 “steps” ). So it takes a long time before mature granulocytes are released into the blood stream where diagnosis occurs (usually by chance since the disease is largely asymptomatic). This explains not only why CML progenitors persist for years in the absence of LSC, but also why the disease is so slow to develop (thus the name “chronic”).
A prediction from this would be that progenitor cells in the blood of imatinib-treated patients would divide much less than those of non-treated patients (since imatinib pushes them into a quicker differentiation). And in fact, telomeres – a chromosomal structure that diminishes as cells divide – are much longer in treated patients supporting the validity of the theory.
The next step – now that progenitors seem to be at the core of CML (and of its cure) – was to understand better their expansion so to better control it.
The answer to Lenaerts and colleagues resided in the different populations’ fitness (their capacity to produce descendants - the more they reproduce, the higher the fitness), since CML outcome will depend on which population - leukemic, imatinib-treated leukemic or normal progenitors - out-competes the others.
Previous studies have shown that leukemic progenitors had higher fitness than normal progenitors (agreeing with the fact that they divide so much that fill the BM), while imatinib-treated leukemic progenitors had the lowest fitness of all. This means that normal progenitors will out-compete leukemic-treated ones, and can even eliminate them if BRC-ABL inhibitors - like imatinib - are given in enough quantities and for long enough.
Using this information Lenaerts and colleagues were able to calculate that about 3.5 years of treatment should be enough to get rid of all molecular traces of BCR-ABL (slightly longer in patients still with LSC at the time of the treatment but, even in those, 80% should have no traces after 6 years). Again, and crucial to the validity of the research, these predictions agree with what was seen on patients under imatinib treatment.
In conclusion, Lenaerts ’ work shows that CML development (including its response to therapy) depends mostly of progenitors cells and that these are the cells to target. And that the key to a cure lays in drugs, like imatinib, which control their expansion (helping normal progenitors to outnumber and, eventually, eradicate them). They also suggest how to improve the chances of a cure: imatinib should start as early as possible, be given for longer than what is used now and maybe at higher doses since the drug is well tolerated and current quantities apparently affect only 5% of all leukemic cells. A crucial conclusion from the new study is the fact that current treatments have the potential to cure the disease
The research can also explain why drugs that suppress cell division are not efficient treating CML, as they are not able to discriminate between leukemic and non leukemic cells.
A physicist and a medical doctor respectively, Jorge Pacheco and David Dingli have worked together several times before – the study in this article is just the latest example – trying to unravel the intricacies behind biological systems, particularly cancer, using a mathematical models, with remarkable results and even more interesting suggestions to change our approach to the disease and its treatments. They believe that more than kill all cancer cells (which tends to carry side-effects besides being many times a feat impossible to guarantee) a better approach is to help healthy cells outcompete and eradicate them. This approach, after all, has serve well cancer cells, which are known to multiply more and die less than normal cells (both these characteristics are the hallmark of cancer) outnumbering healthy cells until there is no space or resources for normal tissues to survive. It is this very different holistic approach to disease (and its success answering difficult questions) that makes this their work so interesting.
1 Haematologica – Advance Online Publication doi:10.3324/haematol.2009.015271. Tyrosine kinase inhibitor therapy can cure chronic myeloid leukemia without hitting leukemic stem”
http://www.haematologica.org/cgi/reprint/haematol.2009.015271v1






Comments