As we reach old age, our bodies undergo several changes. And not for the better. More and more people are beginning to wonder whether we can do something about this. A lot of research is going on to uncover the mechanisms of aging, which should give us a better understanding of the origin of the changes that occur. Some claim that with this understanding comes the increasing possibility that we will actually be able to do something about it.
Now, a new study has shown that the removal of senescent cells can delay aging. As cells age, they accumulate DNA damage. This, in turn, leads to cellular senescence and cell death, both processes that are thought to be important contributors to the aging process. In senescent cells, a certain protein (p16lnk4a) increases in concentration, enforcing cell-cycle arrest.
The research team used transgenic mice to establish the role of senescent cells in aging, and took a look at the effects of eliminating the cells which expressed the aforementioned protein. The researchers engineered mice so that the cells that would express the protein could be selectively targeted by drugs, effectively killing the cells. These mice were intercrossed with others, engineered to express low levels of the BubRr1 protein, which leads to premature development of age-related disorders. In the offspring, the cells expressing the p16lnk4a protein were specifically targeted and killed (see figure 1).
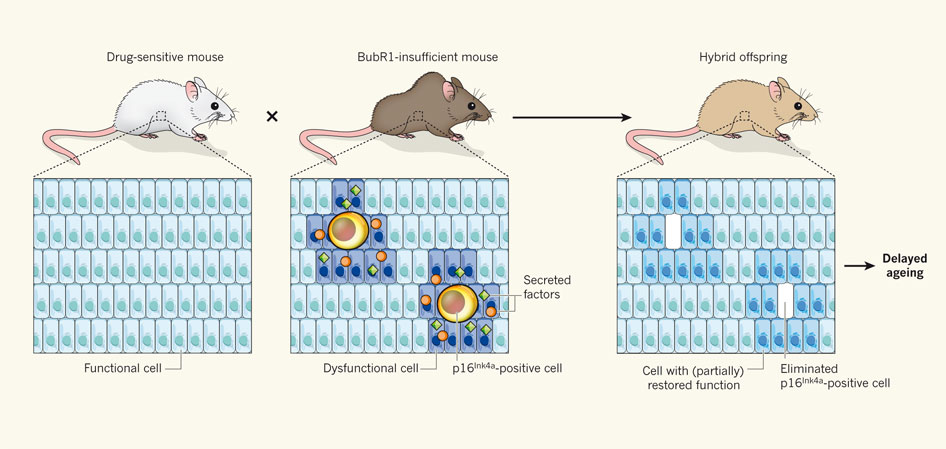
Figure 1: Experimental procedure used by Baker et al. (2011).
(Source: Peeper, 2011)
The elimination of these cells resulted in a delay of age-related changes in the offspring. And more, compared to control mice, they showed thicker muscle fibers, and a greater exercise fitness. Of course, it would be interesting to see whether the removal of senescent cells had any effects after mice had begun to show signs of aging.
And that’s exactly what the team did. In a group of five-month old mice, which begin showing signs of aging, the senescent cells were removed. Five months later, the mice showed thicker muscle fibers and greater fitness, along with a decreased level of senescence markers than a group of control mice.
The authors conclude:
There were no overt side effects of senescent cell clearance in our model, even though it has been postulated that senescent cells enhance certain types of tissue repair. Our proof-of-principle experiments demonstrate that therapeutic interventions to clear senescent cells or block their effects may represent an avenue for treating or delaying age-related diseases and improving healthy human lifespan.
In the same issue of Nature, a view on the study is published. Some interesting points and questions are stated there:
Baker and colleagues' elegant work is a proof-of-concept for the premise that therapeutic elimination of senescent cells may delay ageing-associated processes. Among the questions that therefore arise are whether these findings can be extrapolated to natural ageing, and whether the observed functional improvements are due to termination of further damage or damage reversal.
For therapeutic purposes, regulating the effects of senescent cells, rather than their abundance, may be more manageable. Of relevance to this is the large number of factors that are secreted by senescent cells. Some of these reinforce senescence in their cell of origin, whereas others affect nearby and even distant cells, disrupting vital processes such as tissue function and regeneration. So identifying the factors that are secreted by senescent cells and contribute to ageing is a priority.
Deficiency of p16Ink4a ameliorates traits of premature ageing, but does not completely prevent them or extend lifespan. Consistent with this, Baker et al. showed that features of ageing that are independent of p16Ink4a remain unchanged, supporting the notion that additional factors contribute to premature ageing. Investigations are therefore needed into whether such factors are directly involved in senescence, or instead are effector molecules of BubR1 function (such as the anaphase-promoting complex).
This paper may also provide a clue to why some organisms live longer than others. A provocative thought is that long-lived organisms may be equipped with an immune system that is better at eradicating senescent cells.
And it concludes:
It was recently shown that reactivation of the enzyme telomerase reverses degenerative traits. Baker et al. now find that clearing senescent cells delays age-related dysfunction in the setting of BubR1 insufficiency. Surely, researchers will now keenly explore these and other routes for targeting cellular senescence, in search of a viable strategy that would allow us to get older more healthily.
References
Baker, D.J.; Wijshake, T.; Tchkonia, T.; LeBrasseur, N.K.; Childs, B.G.; van de Sluis, B.; Kirkland, J.L. and van Deursen, J.M. (2011). Clearance of p16lnk4a-positive senescent cells delays ageing-associated disorders. Nature. 497, pp. 232- 236. doi:10.1038/nature.10600.
Peeper, D.S. (2011). Ageing: Old cells under attack. Nature. 497, pp. 186 – 187. doi:10.1038/nature.479186a.




Comments