Pretty much everything happening in the brain would fail without astrocytes. These star-shaped glial cells are known to have a critical role in synapse creation, nervous tissue repair, and the formation of the blood-brain barrier. But while we have decades of data in mice about these nervous system support cells, how relevant those experiments are to human biology (and the success of potential therapies) has been an open question.
In Neuron on December 10, Stanford researchers present the first functional and molecular comparison of human and mouse astrocytes, and while 85%-90% of the genes are similar, human astrocytes have unique genes and respond differently to neurotransmitters, particularly glutamate. This presumably means that, at the adult stage, human astrocytes, in contrast to mouse astrocytes, are better at detecting neuroactivity and adjusting their functions in response.
"We are only beginning to understand the unique properties of human astrocytes," says first author Ye Zhang, a postdoctoral scholar in Stanford University School of Medicine's Department of Neurobiology. "We found hundreds of genes expressed exclusively by human astrocytes, and future studies will likely reveal additional biological differences. Potentially, this work will help us recognize the role of these cells in biological disorders."
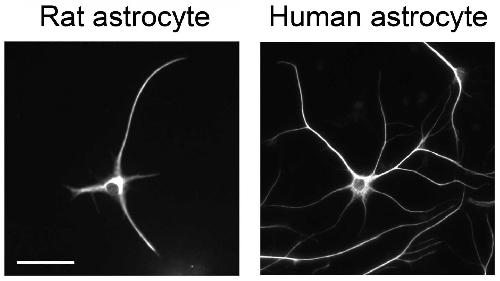
The study of human astrocytes has faced issues related to access (samples of living tissue must be obtained from brain cancer or epilepsy surgeries or fetal tissue) and purification (breaking apart astrocytes away from other cells often killed them and many experiments ended in failure). Zhang, co-first author and graduate student Steven Sloan, and their faculty mentor, senior author and professor Ben Barres, overcame the technical challenges by developing an antibody-driven protocol that isolates astrocytes and keeps them alive in culture.
This method also allowed the researchers to compare astrocytes in healthy tissue versus those coming from people with glioblastoma or epilepsy. It's known from mouse studies that astrocytes become highly reactive in these diseases, but what this means remains unclear. Genes that produce both positive and negative effects are expressed during these active periods, and through this study, some of the good and bad genes in humans are beginning to be parsed out. The next step is to screen for drugs that can promote or quell the expression of specific genes.
Another surprising discovery was that astrocytes come in two distinct stages (progenitor and mature) and that early-stage astrocytes and brain cancer closely resemble one another. This brings up the possibility that brain cancer cells that originate from glial cells can be forced into a mature state and thus unable to divide. The authors note that this finding could not have been made without the use of fetal tissue.
"Such knowledge could not have been obtained without access to fetal tissue," Zhang says. "We can't guess the biology of human brains and neurodevelopmental disorders just by studying mouse brains."
With their new method, Zhang and her colleagues hope to soon begin looking at the unique properties of human astrocyte cells in a range of disease types, including Alzheimer's, ALS, stroke, injury, autism, and schizophrenia.






Comments