Even in healthy people, stem cells in the blood routinely accumulate new mutations over the course of a person’s lifetime. In many cases only two or three additional genetic changes are required to transform a normal blood cell already dotted with mutations into acute myeloid leukemia (AML).
AML is a blood cancer that develops when too many immature blood cells crowd out the healthy cells. In recent years, researchers sequenced the genomes of 200 patients with AML to try to understand the mutations at the root of the disease. Without fail, each patient’s leukemia cells held hundreds of mutations, posing a conundrum for scientists, who have long believed that all the mutations in a cancer cell are likely to be important for the disease to progress.
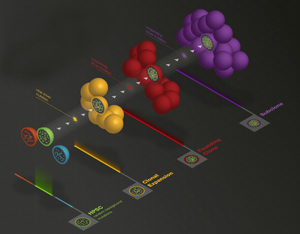
Stem cells in the blood accumulate hundreds of mutations, even in healthy people. When a cancer-initiating event occurs in one of these stem cells, it captures the genetic history of that cell, including the earlier mutations, and drives leukemia to develop. A schematic of that evolution is pictured above. Credit: Joshua McMichael, The Genome Institute
“Now we have a very accurate picture of how acute leukemia develops,” says senior author Richard K. Wilson, PhD, director of The Genome Institute at Washington University. “It’s not hundreds of mutations that are important but only a few in each patient that push a normal cell to become a cancer cell. Finding these mutations will be important for identifying targeted therapies that can knock down a patient’s cancer."
“But we knew all of these mutations couldn’t be important,” says co-first author Daniel Link, MD, professor of medicine at Washington University. “It didn’t make any sense to us that so many mutations were present in all the cells in the tumor.”
To investigate the origin of these mutations, the researchers isolated blood stem cells from healthy people of different ages. The youngest were newborns, and the oldest was in his 70s.
Every person has about 10,000 blood stem cells in their bone marrow, and the researchers found that each stem cell acquires about 10 mutations over the course of a year. By age 50, a person has accumulated nearly 500 mutations in every blood stem cell.
“Mutations are known to develop in cells as we age, but no one had any idea how many mutations occur in blood stem cells and how frequently they develop,” Link says. “These random, background mutations occur during cell division and are unrelated to cancer. Our DNA can tolerate a huge number of these hits without any negative consequences. But if a cancer-initiating event occurs in one of these stem cells, it captures the genetic history of that cell, including the earlier mutations, and drives leukemia to develop.”
As part of the study, the researchers sequenced the genomes of 24 patients with AML and compared the mutations in their leukemia cells to those that occurred in the blood stem cells of the healthy individuals. The scientists were surprised to see that the total number of mutations varied by age, not by whether a patient had leukemia. Thus, a healthy person in his 40s had just about the same number of mutations in his blood stem cells as a leukemia patient of the same age had in his cancer cells.
The study’s results help to explain why leukemia occurs more frequently as people age.
“AML is relatively uncommon until about age 60,” says co-first author John Welch, MD, PhD, assistant professor of medicine. “It is the persistent, random accumulation of mutations in blood stem cells that contributes to the risk of the disease.”
By sequencing the genomes of the AML patients, the researchers also were able to identify 13 novel “driver” mutations that are likely to be important for leukemia to develop in other patients. They also identified a number of additional cooperating mutations that work together with the driver mutations to give blood stem cells a growth advantage over other cells. In many patients, it appeared that in addition to an initiating driver mutation, only a one or two additional cooperating mutations were important for cancer to occur.
While the findings are important to leukemia, they may also hold true for other cancers.
“Our study does not provide proof that this model applies to other cancers,” says corresponding author Timothy Ley, MD, professor of medicine and of genetics. “But this research suggests that scientists should look. This model could explain the large numbers of mutations we and other researchers are finding in breast, lung and other cancers. The idea that the vast majority of mutations occur in a cell before it becomes cancer is completely novel and should be explored further.”
Published in Cell





Comments