Neutrophils are a type of white blood cell and are summoned to fight infections or injury in any tissue or organ in the body, regardless of cellular and biochemical composition.
How do neutrophils, the body's all terrain vehicles, move in these confined spaces?
A team from Brown University's School of Engineering and the Department of Surgery in the Warren Alpert Medical School collaborated find out. Their technique involved two hydrogel sacks sandwiched together with a minuscule space in between. Neutrophils could be placed in that space, mimicking the confinement they experience within tissue. Time-lapse cameras measure how fast the cells move, and traction force microscopes determine the forces the cells exert on the surrounding gel.
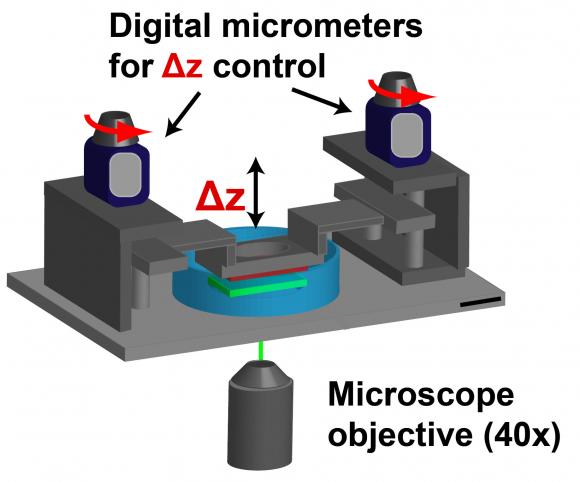
Cell movement in 3-D tissue. By placing neutrophils between two hydrogel sacks, researchers can mimic cell movement through 3-D tissue. Digital micrometers can change the characteristics — density, stiffness — of the medium through which the cells move. Frank lab/Brown University
In a paper published in the Journal of Biological Chemistry, the researchers used the device to reveal new details about the motion of neutrophils. Bodily tissues are highly confined, densely packed, three-dimensional spaces that can vary widely in physical shape and elasticity.
The researchers showed that neutrophils are sensitive to the physical aspects of their environment: They behave differently on flat surfaces than in confined three-dimensional space. Ultimately, the team hopes the system can be useful in screening drugs aimed at optimizing neutrophils to fight infection in specific tissue types.
Traditionally, research on neutrophil motion in the lab is often done on two-dimensional, inflexible surfaces composed of plastic or glass. Those studies showed that neutrophils move using arm-like appendages called integrins. The cell extends the integrins, which grab onto to flat surfaces like tiny grappling hooks. By reeling those integrins back in, the cell is able to crawl along.
Scientists thought that by inhibiting integrins, they could greatly reduce the cells' ability to move through tissue. That, they thought, could be a good strategy for fighting autoimmune diseases in which neutrophils attack and damage healthy tissue.
But in 2008, a landmark paper showed that neutrophils have a second mode of motion. The work showed that cells in which integrins had been disabled were still able to move through dense tissue.
Christian Franck, assistant professor of engineering at Brown, and his colleagues wanted to learn more about this second mode of motion.
"On flat 2-D surfaces there's integrin-dependent motion, but in complicated 3-D materials there's integrin-independent motion," Franck said. "The question we were asking is can we find an in-vitro system that can recreate that integrin-independent motion, because you can't get it in a regular petri dish."
Using their gel system and the traction force microscopes, Franck and his colleagues showed that, when confined, neutrophils exert force in several distinct spots. On the bottom of the cell, forces were generated in a way that was consistent with previous imaging of integrin engagement. But on the top of the cell, there was another source of force. The cell pushed on the upper gel surface with its nuclear lobe, the area of the cell where DNA resides.
"It's like a rock climber pushing against the walls of a canyon," Franck said.
To see if the force generated by the nuclear lobe was responsible for the cells' ability to move without integrins, the researchers repeated the experiment with cells in which integrins were chemically inhibited. Sure enough, the cells were still able to move when confined between the gels. In fact, they were able to move faster.
"We showed that physical confinement is the key feature to reproduce integrin-independent motion in a relatively simple setting," Franck said. "That wasn't possible previously on a flat surface."
The fact that confined cells actually move faster without their integrin suggests that even though integrins aren't essential for the cells motion, they still play a regulatory role.
"What we showed was that [use of integrins] is not black and white," Franck said. "Even in this integrin-independent motion, integrins remain to regulate motion and force generation."
Now that they have a means of recreating how neutrophils travel through confined spaces in the lab, Franck and his team plan to do further experiments aimed at fine-tuning that motion. The system they've developed enables them to control the stiffness of the gel surfaces between which the cells travel, mimicking the varying stiffness of tissue in the body.
"If motility is specific to a neutrophil being in a specific tissue, maybe we could attenuate its response," Franck said. "Maybe we could make it move faster in the muscle and slower everywhere else, for example."
This new system enables testing of drugs aimed at doing just that. Such drugs could be of great benefit to people who have disorders of the immune system.
Franck’s co-authors on the study were Jennet Toyjanova, Estefany Flores-Cortez, and Jonathan S. Reichner. The research was supported by the National Institutes of Health (grants GM066194 and AI101469), and by a Brown University seed grant. Source: Brown University






Comments