Dr. Deutsch and colleagues in the Departments of Biochemistry and Cell Biology (Martin Kaczocha) and Neurobiology and Behavior (Sherrye Glaser, Ph.D.) are the first to successfully identify two known fatty acid binding proteins (FABPs) that carry the endocannabinoid anandamide (AEA), a neurotransmitter, from the cell membrane to interior of the cell where it is destroyed. This identification enabled the research team to inhibit FABPs in various laboratory experiments and thereby reduce AEA breakdown inside cells.
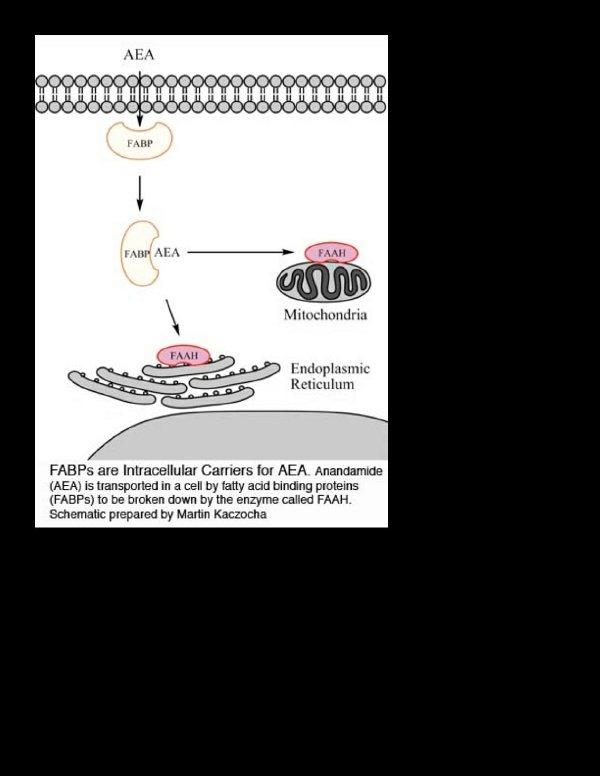
Path of FABPs as intracellular carriers for AEA. Credit: Martin Kaczocha, Stony Brook University
In their study, “Identification of intracellular carriers for the endocannabinoid anandamide,” the researchers report that they decreased the breakdown of AEA in some instances by approximately 50 percent.
“Inhibiting FABPs could potentially raise the levels of AEA in the brain’s synapses,” says Dr. Deutsch. “Naturally occurring AEA levels have been shown to curb pain without the negative side effects, such as motor coordination problems, from molecules like THC. Therefore, it makes sense to target AEA for therapeutic purposes.”
He emphasizes that their groundbreaking discovery of the role of FABPs in transporting this class of neurotransmitters may prove to be a crucial step in developing novel drug targets for endocannabinoids by way of inhibiting FABPs. In support of the research, The State University of New York (SUNY) Stony Brook Office of Technology Licensing and Industry Relations (OTLIR) has filed U.S. Patent applications comprising the discovery.
The OTLIR manages all intellectual property matters for the SUNY Research Foundation. In actively marketing this unlicensed technology created by Dr. Deutsch, the Stony Brook OTLIR welcomes commercial entities interested in partnering with the University. The licensing agent for the project is Adam DeRosa of the OTLIR.
The breakdown of AEA requires two factors. First, there needs to be a mechanism for transporting AEA to the location where it is inactivated because AEA is a fatty compound and thus unable to move inside the watery cellular environment. Second, the cell must express an enzyme called FAAH, which controls the breakdown and inactivation of AEA. In the laboratory, the researchers coaxed a nonneuronal cell type (COS-7) to express FAAH. These FAAH-expressing COS-7 cells were able to break down AED efficiently, indicating that the intracellular AEA transport mechanism was already present and operation in these cells. The researchers identified these carriers as two separate FABPs.
Dr. Deutsch believes that because a transporter for the AEA class of neurotrasmitters had never been discovered until the Stony Brook findings, continued research may explain many unanswered questions about AEA. Future research may uncover more knowledge about AEA transport, as well as the entire role these neurotransmitters play in pain, inflammation, appetite control, addiction, and perhaps other physiological processes related to many human disorders.
The research was funded by the National Institute on Drug Abuse, part of the National Institutes of Health.






Comments