Methane, a greenhouse gas with an impact over 20 times greater than CO2, is constantly seeping out of large methane hydrate reservoirs in the ocean floors but 80 percent of it is immediately consumed by syntrophic ("feeding together") microorganisms.
These microorganisms dramatically reduce the oceanic emission of methane into the atmosphere by oxidizing methane anaerobically, providing an important component of the global carbon cycle and a major sink for methane on Earth.
Scientists of the Helmholtz Centre for Environmental Research (UFZ) in Leipzig and the California Institute of Technology (Caltech) in Pasadena succeeded in capturing these syntrophic microorganisms, something various international research groups have been working on since 1999.

They developed a new molecular technique (US Patent 11/746,374 Pernthaler A, Orphan VJ, 2007) to selectively separate these microorganisms from their natural complex community and subsequently sequenced their genome.
The findings were exciting: Besides identifying all genes responsible for the anaerobic oxidation of methane, new bacterial partners of this syntrophic association were discovered and the ability to fix N2 could be demonstrated.
Microorganisms are the unseen majority on our planet: There are more than 100 Million times more microbial cells than stars in the visible universe, accounting for more than 90 percent of the Earth's biomass, yet we have little idea what most of these bacteria and archaea are doing.
It is not only their small size that makes them hard to study: Most microorganisms can not be grown in the lab. Recent developments of new molecular techniques allow the study of microorganisms where they live, in nature, and this is leading to an explosion of knowledge with no end in sight.
One of these techniques is genome sequencing - learning about the genetic make-up of an organism. This works well for single organisms, such as the sequencing of the human genome but the complexity of natural microbial communities is a major problem. The vast collection of genes can often not be linked to an organism or a physiological process. This plenitude of general information can be compared to a one-billion pieces puzzle of which you own only 300 pieces and you have to try to find out where which piece belongs and how the whole picture could look like.
Scientist at UFZ and Caltech now developed a method that solves this problem. Pernthaler and co-workers attached small ironbeads to the microorganisms of interest and pulled them out of the deep sea sediment by simply applying a magnet. These microbes are Archaea, which cooperate with sulfate reducing Bacteria to perform a thermodynamically tricky process: the anaerobic oxidation of methane (AOM). These poorly understood consortia are globally distributed in oceanic sediments above methane hydrates and provide a significant sink for methane by substantially reducing the export of this potent greenhouse gas into the atmosphere. After sequencing the genomes of the purified syntrophic consortia, Pernthaler and co-workers could find all genes responsible for AOM.
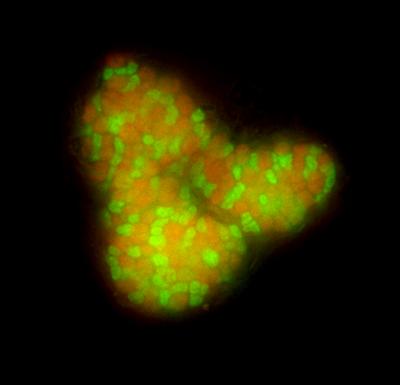
The scientist also discovered an unexpected diversity in the bacterial partners of this syntrophic association, which may play a role in the performance of AOM. Pernthaler and co-workers also found genes for N2 fixation and demonstrated in lab experiments that the AOM archaea are indeed fixing N2.
These results are intriguing, especially since the fixation of N2 is energetically expensive processes and the energy gained by AOM is low. The potential for metabolic versatility combined with the ability to form partnerships with other microorganisms, might be the secret to the successful distribution of this biogeochemically significant group of microorganisms.
Article: Annelie Pernthaler,Anne E. Dekas, C. Titus Brown, Shana K. Goffredi, Tsegereda Embaye, and Victoria J. Orphan, 'Diverse syntrophic partnerships from deep-sea methane vents revealed by direct cell capture and metagenomics', PNAS | May 13, 2008 | vol. 105 | no. 19 | 7052-7057 10.1073/pnas.0711303105






Comments