Structure of the atom
A basic atomic model will suffice for our discussion. In general, we can consider the atom to be composed of protons and neutrons tightly bound within the nucleus of the atom surrounded by electrons. The nucleus comprises almost the entirety of the mass of the atom, while the orbiting electrons make up the size of the atom.
Nuclear reactions
The 2 major types of nuclear reactions are fusion and fission. Most lay people have a rough idea of what this means. Fusion is when 2 or more light nuclei are bonded together to form a heavier atom and by contrast fission is the process whereby a heavy atom separates into 2 lighter atoms. In both these process, a certain amount of energy is released making the reactions exothermic.
Radiation
There are 3 main forms of radiation that the reader needs to be aware of: Alpha, Beta and Gamma. Alpha radiation occurs when an unstable atom releases a Helium 4 nucleus (2 protons&2 neutrons) from its core. Beta radiation are electrons that are released from the atom. This usually occurs in unstable atoms where either a proton or neutron undergoes decay. Gamma radiation is very high frequency electromagnetic radiation that is emitted either as an accompaniment to alpha or beta radiation, or when an excited atom returns to its ground state.
Out of the 3 radiations, gamma can cause the largest risk to humans because it requires large amounts of shielding. Both alpha and beta particles can be shielded from relatively easily, but a significantly thick barrier of concrete or lead might be necessary to completely guard against gamma radiation. I will describe how radiation actually becomes harmful to humans in another article.
Why nuclear power produces energy
Moving along, the above information is sufficient to understand how the fission process occurs. Before getting right into the discussion, I think it is an interesting question to ask why both combining small atoms and splitting large atoms produce excess energy. To answer that question, a graph of Nuclear Binding energy vs atomic mass number needs to be studied. An example of this graph can be found from Wikipedia.
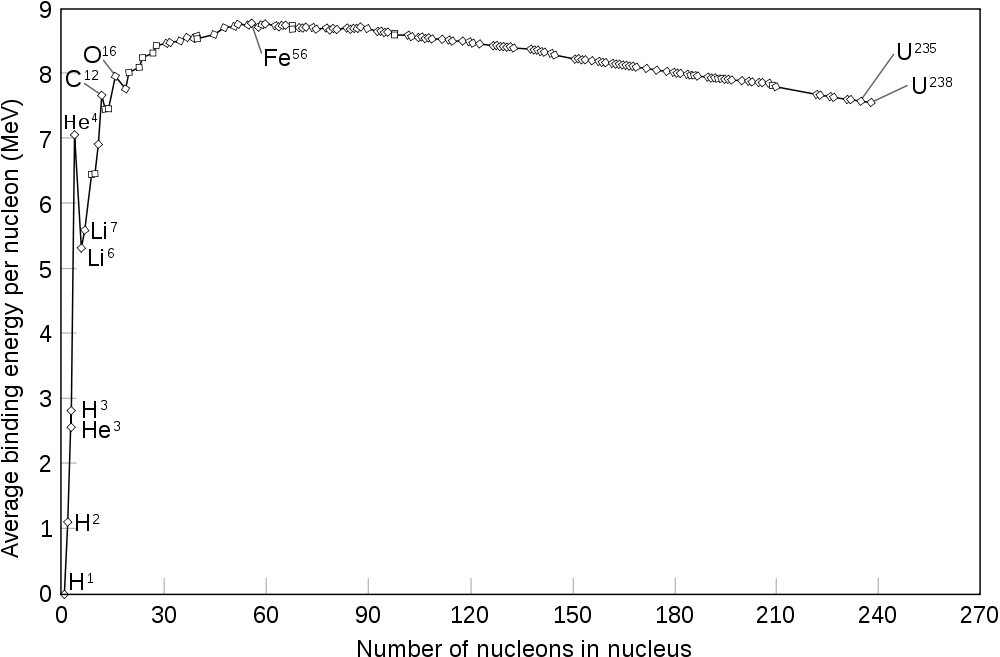
The graph in the link shows how strongly bound the nucleons (protons&neutrons in the nucleus) in an atom is compared to the number of nucleons in the atom. What this graph shows is that as the number of nucleons increases, the more tightly bound each nucleon is to one another approaching a maximum around Iron and Nickel, after which there is a smooth decline of the binding energy. Now, without becoming too technical, the value of the binding energy is proportional to how much energy can be saved by being in that state. I.e. if combining 2 atoms results in the third atom have a higher binding energy than the average of its parts, then energy can be released in the reaction. Alternatively, if in splitting a heavy atom, the average of the binding energy of its' parts is higher than the binding energy of the original nucleus, then energy is released in the reaction.
An interesting analogy is to consider people in a relationship. When a couple first meets up, and they are compatible, then being together takes less energy than being apart - so they tend to be together. If on the other hand, people are bound in the relationship by clutter and junk, then separating and being apart requires less energy than being together. This extra energy that is saved (either by fusing or splitting) is what powers reactors.
Fission
It is important to note that the binding energy does not give an indication of the stability of the atom. To understand stability, we will need to consider the atom in a little more detail. We know that the protons carry a positive charge, and also that like charges repel. It is then counter intuitive that protons remain together in the nucleus. And this is where the mighty neutron comes in. The neutron has no charge and is almost equivalent in mass to a proton. The neutrons acts to binds the nucleons within the nucleus using the strong nuclear force. This overcomes the repulsive force between protons to keep the atom in tact. As the number of protons in an atom increases, the number of neutrons needed to keep the atom stable increases. There is usually an optimal number of neutrons to keep an atom stable. If there are more neutrons or protons in a nucleus than the optimal amount, the atom tends to become unstable (especially for larger atoms).
Now an unstable atom usually undergoes alpha or beta decay or in some circumstances, spontaneous fission. Usually a single radioactive decay is insufficient to produce a stable atom, and a long chain of decays occur where finally a stable atom is reached. Some atoms (like Uranium) have very long half-lives and can be considered semi-stable. These heavy semi-stable isotopes are the most important elements in a nuclear reaction.
A few new concepts need to discussed before going further - and to make these essays readable, I'll stop here for now and continue further in a subsequent article.





Comments