Then you get special dispensation. It’s called the dietary supplement industry. And what they get away with is astounding.
Last May a cluster of liver failure was attributed to a supplement called OxyElite Pro, sold by USPLabs of Dallas.
Of the 29 people identified, 24 had taken this “natural” supplement. Somehow, with a straight face, a corporate spokesperson issued statement: “[USPLabs] stand[s] by the safety of all of its products … the cluster of liver issues in Hawaii is a complete mystery.” That’s a good one.
A new report in The New England Journal of Medicine points out that the problem was actually worse. By this past February, the CDC had found 97 cases of liver damage in people who had used this product. Of this group, 47 people were hospitalized, three needed liver transplants and one died.
So, how did the FDA let this poison get into stores? That’s an easy one—there was nothing they could do to stop it. Instead, they ordered the product to be taken off the market—after the damage had been done.
Whoa! Isn’t this backwards? Isn’t their job to ensure that drugs have been evaluated for safety and efficacy before they are given approval to be sold?
Yes, it is. Except in Bizzaro World, which in this case happens to be Utah. Why? Keep reading.
But, if something is not a drug, then the FDA doesn’t get to review it and cannot approve or reject it. So, the obvious solution is to take a drug and call it something else—a supplement.
Senator Orrin Hatch of Utah—the home of much of the mega-billion dollar supplement industry—pushed through a law laughably called The Dietary Supplement Health and Education Act of 1994. It should have been called the “Doubletalk Doodie in a Bottle” act, because its function was to provide a scientifically meaningless divide between some drugs and other drugs by calling them supplements.
Wait a minute. Supplements aren’t supposed to be drugs, right? After all, they are natural, safe and good for you. No, no,and no. More on that later.
Please explain why anyone would swallow something that didn’t cure, mitigate, treat, or prevent disease—exactly how the FDA defines a drug—if it doesn’t do any of these things. Yet, the industry sells thirty billion dollars worth of it every year.
This is where the doubletalk comes in.The 1994 law, using carefully crafted language, permits some drugs to be declared to be not drugs by considering them to be foods. Which is utter nonsense.
And so is the definition of a supplement, according to this law: a product (other than tobacco)intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite,constituent, extract, or combination of any of the aforementioned ingredients.
Cute. Take a vitamin, amino acid, or some goo from a plant and chuck it in with who-knows-what and you have a food.
Some people sure must have strange diets, since if I’m reading this right; strychnine (rat poison), coniine (hemlock—the stuff that killed Socrates), and urushiol (the active chemical from poison ivy) are all foods. And I thought my cooking was bad.
Irony time: Phenylalanine and aspartic acid are amino acids that are both often attacked as health risks. Why? Because they are two of the three components of aspartame (NutraSweet)—which after decades of use is still called “by far the most dangerous substance on the market that is added to food.” And if you don’t believe me, go to Crazy Joe Mercola’s website and read it yourself.
Yet, you can buy both phenylalanine and aspartic acid on Amazon, where they are sold as—you guessed it—supplements. And if this isn’t hilarious enough, you can also buy glutamic acid (MSG) there too. My sides!
The labeling requirements on supplement bottles are pretty funny too. They must say: “This product is not intended to diagnose, treat, cure, or prevent any disease" if the supplement bears a claim to affect the structure or function of the body (structure/function claim), a claim of general well-being, or a claim of a benefit related to a classical nutrient deficiency disease.”
OK, then tell me why do supplement labels routinely say “Supports X,” where X can be anything like prostate health, your immune system, liver function, sexual well being or memory?
This the sleazy language that allows supplements/aka drugs to be sold without making a medical claim. But when people see “supports” (wink, wink) they sure as hell are thinking that they are buying a product to cure, treat or prevent something or other. Gee—kind of sounds like a drug, no?
Speaking of which, please let me know what DHEA (an anabolic steroid), MSM, aka dimethylsulfone—an industrial solvent— and dimethylamylamine (DMAA)—an amphetamine-like stimulant and the prime suspect in the hepatitis cases are supplementing?
They are supplementing the profit margins of the companies that sell this junk. Let’s put a label on this one—“Supports our bottom line.”
And the problems don’t stop there. Notonly are people buying unregulated drugs at GNC and the Vitamin Shoppe, but theymay not even be getting what they think.
Some common issues:
1. The amount of ingredient(s) in the pill may vary widely from what is on the label
2. An otherwise-useless herbal product may be spiked with a prescription drug—most commonly with erectile dysfunction products.
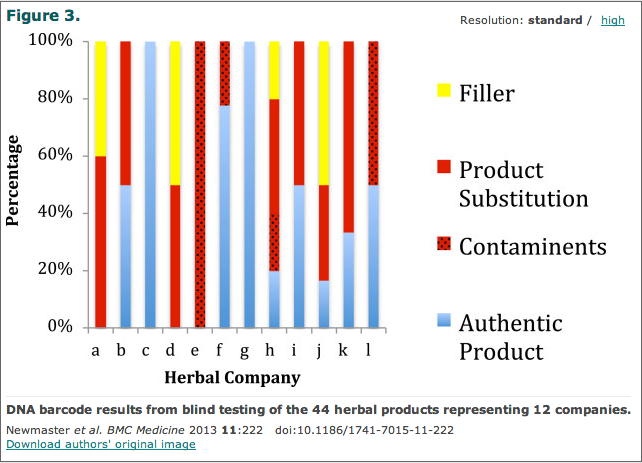
3. By far the worst, what is on the label may not be what is in the bottle.In fact, it rarely is. A damning study published in BMCMedicine looked at contamination of 44 herbal products that were sold by 12different companies. Using DNA technology to identify plant species, the researchers found that 59 percent of the products they tested contained species of plants that were not listed on the label.
Worse still, when they looked at 44 herbal products from 12 different companies, only two of the companies sold what was actually on the label. The rest sold products with various fillers andcontaminants.
What kind of contaminants? Pretty much what you’d expect. Various toxins, a few carcinogens and other icky stuff. For herbal supplement users, the graph below can’t be especially comforting.
Not surprisingly, the authors concluded,“Many of the dangers of commercial plant medicine have been brought to light by DNA technology based studies that have identified contamination of herbal products with poisonous plants.”
Yet, thanks to Senator Hatch, it is perfectly legal to sell this garbage. And the FDA can only stand by and watch for the next poisoning—after it happens. I suspect that they won’t have to wait long.
And this is what people who are afraid of drugs and chemicals are consuming with the expectation that it will somehow benefit them?
Pharmaceutical companies spend hundreds of millions of dollars to determine the safety of new drugs, only to have many of them fail anyhow due to unexpected side effects. But manufacturers of supplements are not held to any regulations that even approach this level of scrutiny.
Supplement regulations are driven by money and sleight-of-hand, not science. But drugs are drugs. They should all be treated the same.





Comments