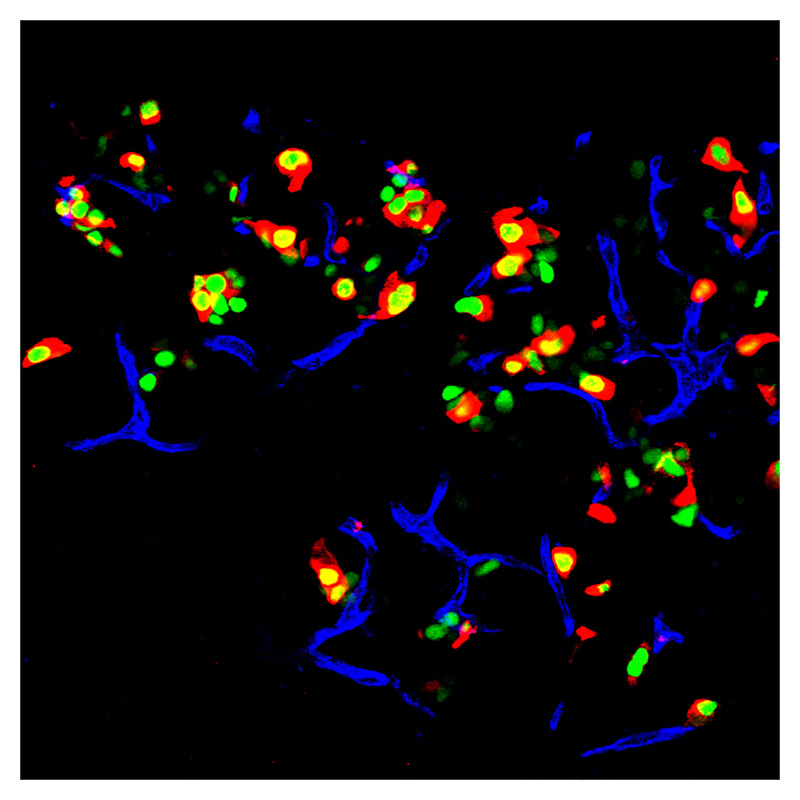
 An immunofluorescent image of an adult mouse pancreas, where exocrine cells are displayed in green. The transcription factors were inserted into these cells. The red areas in the image are insulin. Credit: Qiao Zhou, Melton Laboratory, HHMI at Harvard University.
The group created pancreatic beta cells, a rare cell type which comprises only about one percent of the pancreas. These are the insulin-producing cells that die in Type-I diabetes, and the cells that Melton's lab created can secrete insulin and therefore ameliorate hyperglycemia.
In less technical terms, they were able to change one kind of cell into another; they reprogrammed exocrine cells into beta cells, without having to use stem cells.
This is exciting because it is a parallel way of approaching the same kind of problem that researchers working with induced pluripotent stem cells (iPS) cells deal with: how to induce one kind of cell to differentiate into the other kind that you want. When the process of creating iPS cells was introduced two years ago by Japanese researcher Shinya Yamanaka, one of the main problems involved figuring out how to reprogram the stem cells so that they would actually turn into the kind of cell that was desired.
While there have been some examples of adult cell reprogramming in the literature, there is no general understanding of how to reliably turn one cell type into another.
What is the advantage of using this method over embryonic stem cells or iPS cells?
One of the goals of regenerative medicine is to convert adult cells from patients into other cell types that could be used for tissue repair or to replace cells that are lost and diseased, but Melton explains, “we and others have not been able to produce in any reasonable quantity fully formed pancreatic beta cells from embryonic stem cells or iPS cells. That requires many steps of educating the cells, and we haven’t solved all of those steps."
Before you start referring to the stem cell debate in Washington in the past tense, the research from Melton's lab most likely will not eliminate the need for stem cells.
An immunofluorescent image of an adult mouse pancreas, where exocrine cells are displayed in green. The transcription factors were inserted into these cells. The red areas in the image are insulin. Credit: Qiao Zhou, Melton Laboratory, HHMI at Harvard University.
The group created pancreatic beta cells, a rare cell type which comprises only about one percent of the pancreas. These are the insulin-producing cells that die in Type-I diabetes, and the cells that Melton's lab created can secrete insulin and therefore ameliorate hyperglycemia.
In less technical terms, they were able to change one kind of cell into another; they reprogrammed exocrine cells into beta cells, without having to use stem cells.
This is exciting because it is a parallel way of approaching the same kind of problem that researchers working with induced pluripotent stem cells (iPS) cells deal with: how to induce one kind of cell to differentiate into the other kind that you want. When the process of creating iPS cells was introduced two years ago by Japanese researcher Shinya Yamanaka, one of the main problems involved figuring out how to reprogram the stem cells so that they would actually turn into the kind of cell that was desired.
While there have been some examples of adult cell reprogramming in the literature, there is no general understanding of how to reliably turn one cell type into another.
What is the advantage of using this method over embryonic stem cells or iPS cells?
One of the goals of regenerative medicine is to convert adult cells from patients into other cell types that could be used for tissue repair or to replace cells that are lost and diseased, but Melton explains, “we and others have not been able to produce in any reasonable quantity fully formed pancreatic beta cells from embryonic stem cells or iPS cells. That requires many steps of educating the cells, and we haven’t solved all of those steps."
Before you start referring to the stem cell debate in Washington in the past tense, the research from Melton's lab most likely will not eliminate the need for stem cells.
"We really wouldn’t be where we are today without working with human embryonic stem cells."
Melton emphasized, "We are continuing to do research using hES (human embryonic stem) cells and iPS cells. We really wouldn’t be where we are today without working with hES cells, because they provide a unique window into human development and disease, and we need those to further progress our understanding. "These cells will remain a key part in regenerative medicine, and what we are reporting today is a parallel approach for making cells for regenerative medicine." While Melton's lab successfully reprogrammed these cells in vivo (in a live animal), it may be a few years before this treatment is curing people from diabetes or Parkinson's. Melton explained, "We’ve made the target cell that we want—the beta cell—and now to perfect it in terms of curing diabetes, what we’d like to do is to get a collection of those cells to migrate together in what’s called a 'pancreatic islet.'
"We’ve made the cell type. We just haven’t yet made the whole tissue."
"That has yet to be achieved, but I’m reasonably confident we’ll be able to do that. We’ve made the cell type. We just haven’t yet made the whole tissue." Melton expressed optimism in his outlook for also eventually using this method to treat diseases in other systems like the nervous system or cardiovascular system. "I think this approach could be broadly applicable. We may be able to use a similar approach to help treat neurodegenerative diseases like Parkinson’s or ALS, where there are healthy cells nearby the diseased cells."






Comments