Heart attacks are often caused by conditions that affect electrical signaling in the heart. Genetic studies have linked two of these conditions, long QT syndrome and Brugada syndrome, to mutations in the sodium channels that let sodium ions into cells in response to electrical signals.
A multidisciplinary team has been tracking the complex of proteins thought to be at fault in some cases of sudden cardiac death and now they have finally captured images of the complex. Those images reveal the connection between some genetic mutations and electrical abnormalities of the heart and provide a starting point for designing therapies.
Many of the mutations affect a particular part of the channel that was thought to interact with a protein called calmodulin. This seemed important, because, explains Gordon Tomaselli, M.D., the Michel Mirowski, M.D., Professor of Cardiology at the Johns Hopkins University School of Medicine, "Calmodulin is the most ubiquitous calcium-binding protein in nature, and it regulates the function of sodium channels not only in the heart, but also in the brain and muscle."
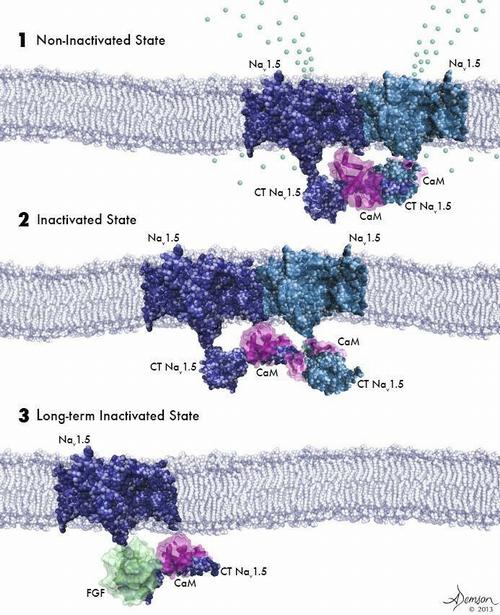
(1) Two activated sodium channels embedded in the cell membrane are bound to each other and to a calmodulin molecule (purple). (2) Sodium channels bound to each other and to calmodulin are poised for activation but have not yet been opened by voltage change. (3) A sodium channel not bound to another channel cannot be activated by a voltage change. © Anastasia Demson, 2014
The genetic studies were a hint that many cases of sudden cardiac death might come down to faulty interactions between sodium channels and calmodulin, which in turn would lead to electrical misfires. In that case, perhaps a drug could be designed that would correct the interaction. But finding such a drug would be a shot in the dark unless researchers could figure out what the sodium channel-calmodulin complex looked like — and how the mutations were affecting it.
Tomaselli's research group teamed with those of L. Mario Amzel, Ph.D., a professor of biophysics and biophysical chemistry, and Sandra Gabelli, Ph.D., an assistant professor of medicine and of biophysics and biophysical chemistry, to try to figure out the structure of the complex and how changes to it affect muscles' electrical properties. To learn that, the researchers had to coax bacterial cells to make human versions of sodium channel proteins and calmodulin, purify the protein complexes, and image them with a technique called X-ray crystallography. "We observed that binding to calmodulin changes the state of the sodium channel, leaving it poised to be activated, or opened, by an electrical signal from outside the cell," Gabelli says.
Previous studies had hinted that poised-for-activation sodium channels tended to be bound not just to calmodulin, but also to a second sodium channel. "As expected, we found that two sodium channels often bind both to each other and to calmodulin," Amzel says. "We were completely surprised, though, to find that it is the same domain of the two channels that interact with each other."The researchers also tested the electrical properties of cells with normal and mutated versions of the sodium channel-calmodulin complex. They found that the latter corresponded with EKG readings of people with long QT and Brugada syndromes.
"Altering calmodulin regulation of sodium channels may be an important and novel therapeutic strategy for the treatment of heart failure, arrhythmias, neurological disorders, such as seizures and autism, and diseases of skeletal muscle," Tomaselli says.






Comments