Researchers have identified new genetic mutations in the gene KCNK3 that can cause pulmonary arterial hypertension, a rare fatal disease characterized by high blood pressure in the lungs.
The
KCNK3
mutations appear to affect potassium channels in the pulmonary artery, a mechanism not previously linked to the condition. Cell culture studies showed that the mutations' effects could be reversed with a phospholipase inhibitor. The effects of the KCNK3 mutations were reversed in cell cultures with an experimental phospholipase inhibitor called ONO-RS-082.
Pulmonary arterial hypertension (PAH) is a progressive disorder characterized by abnormally high blood pressure in the pulmonary artery, which reduces blood flow from the right side of the heart to the lungs. The heart can compensate by pumping harder, but over time this can weaken the heart muscle and lead to right-sided heart failure. Common symptoms of PAH include shortness of breath, dizziness, and fainting. About 1,000 new cases are diagnosed in the United States each year. The disorder is twice as common in females as in males. There is no cure for PAH and few effective treatments. Most patients with PAH die within 5–7 years of diagnosis.
Some cases of PAH are caused by inherited genetic defects. Most of these "familial" cases have been linked to mutations in a gene called BMPR2 (bone morphogenetic protein receptor, type II), which was identified simultaneously in 2000 by two independent research teams, one led by the late Robin Barst and Jane Morse, CUMC researchers. However, the majority of cases are idiopathic in origin (of unknown cause). Other forms of PAH can be triggered by autoimmune diseases, congenital heart defects, infections (such as schistosomiasis), and medications (such as the now-banned diet-drug combination commonly known as fen-Phen).
"The most exciting thing about our study is not that we've identified a new gene involved in pulmonary hypertension, but that we've found a drug that can 'rescue' some mutations," said co-senior author Wendy K. Chung, MD, PhD, associate professor of pediatrics and medicine at Columbia University Medical Center. "In genetics, it's common to identify a gene that is the source of a disease. However, it's relatively rare to find potential treatments for genetic diseases."
Chung and her colleagues discovered the new mutations by sequencing the exomes (the portion of the genome that codes information to make proteins) of families with PAH without identified mutations. KCNK3 mutations were found in 3.2 percent of those with familial disease and in 1.3 percent of those with idiopathic PAH.
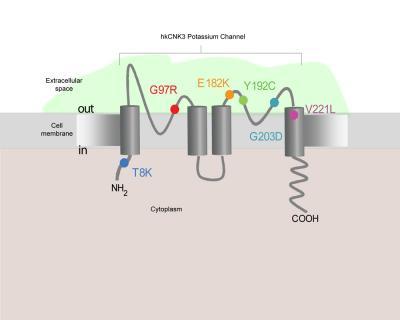
This is a cross section of a potassium channel in a smooth muscle cell of the pulmonary artery. A study by Dr. Wendy Chung has identified six new mutations in a gene called KCNK3 that can interfere with the function of potassium channels and lead to pulmonary hypertension. The mutations are depicted in color at the locations where they exert their effects. Credit: Columbia University Medical Center
The team found that the mutations alter the function of potassium channels by reducing the activity of these channels. Potassium channels help maintain the vascular tone of the pulmonary artery and help it respond to low levels of oxygen.
"We were surprised to learn that KCNK3 appears to play a role in the function of potassium channels in the pulmonary artery," said Chung. "No one had suspected that this mechanism might be associated with PAH." The other gene linked to the disorder, BMPR2, is thought to cause PAH by ultimately promoting growth and multiplication of smooth muscle cells in the pulmonary artery, thereby restricting blood flow.
Further study is needed to determine whether treatment with
ONO-RS-082
or other drugs that affect potassium channels might be useful in the treatment of people with PAH, said Dr. Chung.
"KCNK3 mutations are a rare cause of PAH, so I don't want to oversell our findings," said Dr. Chung. "Still, it's exciting that we've found a mechanism that can lead to the disease that is a new, druggable target. It's also possible that targeting KCNK3 may be beneficial for patients who have PAH independent of their KCNK3 genetic status."
Citation: Lijiang Ma, M.D., Ph.D., Danilo Roman-Campos, Ph.D., Eric D. Austin, M.D., Mélanie Eyries, Ph.D., Kevin S. Sampson, Ph.D., Florent Soubrier, M.D., Ph.D., Marine Germain, M.Sc., David-Alexandre Trégouët, Ph.D., Alain Borczuk, M.D., Erika Berman Rosenzweig, M.D., Barbara Girerd, Ph.D., David Montani, M.D., Ph.D., Marc Humbert, M.D., Ph.D., James E. Loyd, M.D., Robert S. Kass, Ph.D., and Wendy K. Chung, M.D., Ph.D., 'A Novel Channelopathy in Pulmonary Arterial Hypertension', N Engl J Med 2013; 369:351-361July 25, 2013DOI: 10.1056/NEJMoa1211097






Comments