In 1972, a term related to the parts of a genome sequence that don't have a known function was introduced. Like physicists with the 'God particle', a lot of biologists wish they could take back the term 'junk DNA' because it has been colloquialized to mean something different to the public than what it means biologically. Sometimes it just takes time to know what things do, if they do anything. A 2004 Nature paper removed 'gene deserts' - 0.1% of the mouse genome - with no effect they could find.
MicroRNAs are small pieces of genetic material similar to the messenger RNA that carries protein-encoding recipes from a cell's genome out to the protein-building machinery in the cytoplasm. Only microRNAs don't encode proteins so biologists placed the regions of the genome that encode these small, non-protein coding RNAs in the junk category. Now, it is known that although microRNAs don't encode their own proteins, they do bind messenger RNA, preventing their encoded proteins from being constructed. In this way, microRNAs play important roles in determining which proteins are produced (or not produced) at a given time.
MicroRNAs are increasingly recognized as an important part of both normal cellular function and the development of human disease. So, why not embryonic development, too?
An embryo is an amazing thing- from just one initial cell, an entire living, breathing body emerges, full of working cells and organs. Embryonic development is a carefully orchestrated process.
In a study , researchers discovered that microRNAs play an important role in this cell- and germ layer-directing process during development.
"One of the first, and arguably most important, steps in development is the allocation of cells into three germ layers—ectoderm, mesoderm, and endoderm—that give rise to all tissues and organs in the body," says Mark Mercola, Ph.D., professor and director of Sanford-Burnham's Muscle Development and Regeneration Program in the Sanford Children's Health Research Center.
To pinpoint which, if any, microRNAs influence germ layer formation in early embryonic development, Mercola and his team individually studied most (about 900) of the microRNAs from the human genome. They tested each microRNA's ability to direct formation of mesoderm and endoderm from embryonic stem cells. In doing so, they discovered that two microRNA families—called let-7 and miR-18—block endoderm formation, while enhancing mesoderm and ectoderm formation.
The researchers confirmed their finding by artificially blocking let-7 function and checking to see what happened. That move dramatically altered embryonic cell fate, diverting would-be mesoderm and ectoderm into endoderm and underscoring the microRNA's crucial role in development.
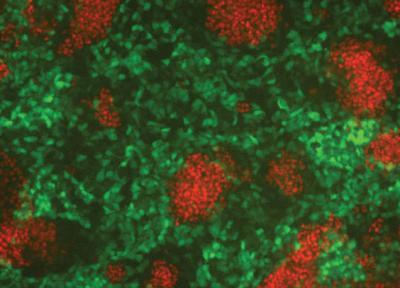
These are differentiating mouse embryonic stem cells (green = mesoderm progenitor cells, red = endoderm progenitor cells). The microRNAs identified in this study block endoderm formation, while enhancing mesoderm formation. Credit: Sanford-Burnham Medical Research Institute
But they still wanted to know more…how do let-7 and miR-18 work? They went on to determine that these microRNAs direct mesoderm and ectoderm formation by dampening the TGFβ signaling pathway. TGFβ is a molecule that influences many cellular behaviors, including proliferation and differentiation. When these microRNAs tinker with TGFβ activity, they send cells on a certain course—some go on to become bone, others brain.
"We've now shown that microRNAs are powerful regulators of embryonic cell fate," Mercola says. "But our study also demonstrates that screening techniques, combined with systems biology, provide a paradigm for whole-genome screening and its use in identifying molecular signals that control complex biological processes."





Comments