The push-and-pull of cellular forces drives the deformation, extension and contraction of cells that occur during tissue development and these processes ultimately shape the architecture of tissues so how it is done plays an important role in coordinating cell signaling, gene expression and behavior, and they are essential for wound healing and tissue homeostasis in adult organisms.
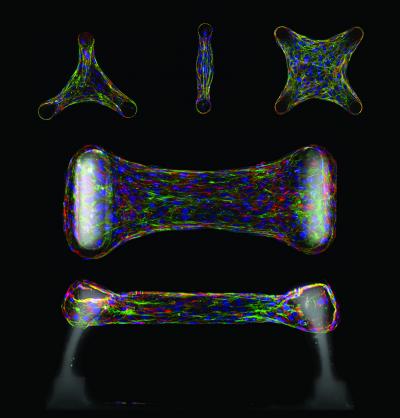
Multicellular architecture regulates cellular function. Immunofluorescence sections of cells embedded within a micropatterned collagen gel. Cells generate forces within a tissue that can feedback to regulate the accumulation of cytoskeletal proteins such as actin (green) and structural matrix proteins such as fibronectin or tenascin C (red). These forces can be measured using elastomeric cantilevers (white) that deflect in response to tissue forces. Cell nuclei are labeled in blue. Credit:Wesley R. Legant
Yet a detailed picture of how tissue mechanics link to morphogenetic phenomena has been hindered by a lack of model systems in which both mechanics and remodeling can be simultaneously examined.
The new Penn study published in PNAS highlights a complex and dynamic relationship between cellular forces, visualizes the remodeling of a matrix by living cells and demonstrates a system to study and apply this relationship within engineered 3-D microtissue.
Chris Chen, professor of bioengineering in the School of Engineering and Applied Science at Penn, developed the tool with colleagues at the University of California, Santa Barbara, and the University of Cambridge.
The system was created using photolithography, the same technology used to craft semiconductors. Scientists fabricated an array of tiny divots within a mold and immersed the mold in a culture of cells and collagen. Researchers then placed raised microcantilever posts on either side of the mold and — much like draping a volleyball net across two metal poles -- observed the formation of a cell and collagen web of living tissue anchored to the cantilevers. These microcantilevers were used to simultaneously constrain the remodeling of a collagen gel and to report forces generated during this process.
Microfabricated tissue gauges to measure and manipulate forces from 3-D microtissues. Video shows time lapse photography of a cell and collagen mix as it retracts and forms tissue. Credit: Image courtesy of Wesley R. Legant, the University of Pennsylvania.
The cantilever posts allowed the team to observe and measure the retraction and extension of the cells as they remodeled the adjacent matrix into a coherent band of tissue. Varying the mechanical stiffness of the cantilevers and collagen matrix demonstrated that the cellular forces increased with boundary or matrix rigidity, whereas the levels of proteins in the cytoskeleton and extracellular matrix also increased with levels of mechanical stress.
By mapping these relationships between cellular and matrix mechanics, cellular forces and protein expression onto a bio-chemo-mechanical model of microtissue contractility, the team demonstrated how intratissue gradients of mechanical stress can emerge from collective cellular contractility and, finally, how such gradients can be used to engineer protein composition and organization within a 3-D tissue.
"Just as we build muscle in the gym, these same mechanical forces are translated down to the cellular level and build the complex arrangement of different tissues in the body," co-author Wesley Legant said. "By varying the properties of our model system, we can study how these mechanical factors are distributed throughout a tissue and how this can, in turn, effect cellular function."
"With this system, we also see the potential for high-throughput drug testing, as researchers will be able to test new pharmaceuticals against a vast array of these small tissue samples, perhaps identifying new ways to increase the contractility of cardiac muscle, or to relax arteries to treat hypertension," said Chen, the study's lead author.
Working with colleagues, the team also created a mathematical model of the entire process that accurately predicted the experimental results.
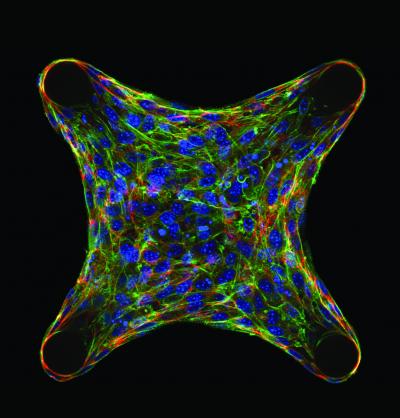
Multicellular architecture regulates cellular function. Immunofluorescence sections of cells embedded within a micropatterned collagen gel. Cells generate forces within a tissue that can feedback to regulate the accumulation of cytoskeletal proteins such as actin (green) and structural matrix proteins such as fibronectin or tenascin C (red) in a spatially dependent manner. Cell nuclei are labeled in blue. Credit: Wesley R Legant
"With this model, we can extend our findings to more complex and realistic model tissues which might be difficult to study experimentally in the lab" Legant said.
The study was conducted by Chen, Legant and Michael T. Yang of the Department of Bioengineering at Penn; Amit Pathak and Robert M. McMeeking of the Department of Mechanical Engineering at UCSB; and Vikram S. Deshpande of the Department of Engineering at Cambridge.
The research was funded by grants from the National Institutes of Health, an Army Research Office Multidisciplinary University Research Initiative, the Material Research Science and Engineering Center and Center for Engineering Cells and Regeneration at Penn, the U.S Department of Education's Graduate Assistance in Areas of National Need and the National Science Foundation's Graduate Research Fellowship.






Comments