Artificial photosynthesis for the production of liquid fuels offers the promise of a renewable and carbon-neutral source of transportation energy, meaning it would not contribute to the global warming that results from the burning of oil and coal. The idea is to improve upon the process that has long-served green plants and certain bacteria by integrating into a single platform light-harvesting systems that can capture solar photons and catalytic systems that can oxidize water – in other words, an artificial leaf.
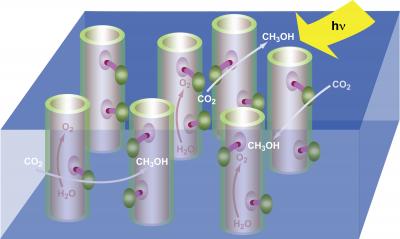
Under the fuel through artificial photosynthesis scenario, nanotubes embedded within a membrane would act like green leaves, using incident solar radiation (Hγ) to split water molecules (H2O), freeing up electrons and oxygen (O2) that then react with carbon dioxide (CO2) to produce a fuel, shown here as methanol (CH3OH). The result is a renewable green energy source that also helps scrub the atmosphere of excessive carbon dioxide from the burning of fossil fuels. Credit: Robert Flavio, Berkeley Lab Public Affairs
"Photooxidation of water molecules into oxygen, electrons and protons (hydrogen ions) is one of the two essential half reactions of an artifical photosynthesis system - it provides the electrons needed to reduce carbon dioxide to a fuel," said Heinz Frei, a chemist with Berkeley Lab's Physical Biosciences Division, who conducted this research with his postdoctoral fellow Feng Jiao. "Effective photooxidation requires a catalyst that is both efficient in its use of solar photons and fast enough to keep up with solar flux in order to avoid wasting those photons. Clusters of cobalt oxide nanocrystals are sufficiently efficient and fast, and are also robust (last a long time) and abundant. They perfectly fit the bill."
Green plants perform the photooxidation of water molecules within a complex of proteins called Photosystem II, in which manganese-containing enzymes serve as the catalyst. Manganese-based organometallic complexes modeled off Photosystem II have shown some promise as photocatalysts for water oxidation but some suffer from being water insoluble and none are very robust. In looking for purely inorganic catalysts that would dissolve in water and would be far more robust than biomimetic materials, Frei and Jiao turned to cobalt oxide, a highly abundant material that is an an important industrial catalyst. When Frei and Jiao tested micron-sized particles of cobalt oxide, they found the particles were inefficient and not nearly fast enough to serve as photocatalysts. However, when they nano-sized the particles it was another story.
"The yield for clusters of cobalt oxide (Co3O4) nano-sized crystals was about 1,600 times higher than for micron-sized particles," said Frei, "and the turnover frequency (speed) was about 1,140 oxygen molecules per second per cluster, which is commensurate with solar flux at ground level (approximately 1,000 Watts per square meter)."
Frei and Jiao used mesoporous silica as their scaffold, growing their cobalt nanocrystals within the naturally parallel nanoscale channels of the silica via a technique known as "wet impregnation." The best performers were rod-shaped crystals measuring 8 nanometers in diameter and 50 nanometers in length, which were interconnected by short bridges to form bundled clusters. The bundles were shaped like a sphere with a diameter of 35 nanometers. While the catalytic efficiency of the cobalt metal itself was important, Frei said the major factor behind the enhanced efficiency and speed of the bundles was their size.
"We suspect that the comparatively very large internal area of these 35 nanometer bundles (where catalysis takes place) was the main factor behind their increased efficiency," he said, "because when we produced larger bundles (65 nanometer diameters), the internal area was reduced and the bundles lost much of that efficiency gain."
Frei and Jiao will be conducting further studies to gain a better understanding of why their cobalt oxide nanocrystal clusters are such efficient and high-speed photocatalysts and also looking into other metal oxide catalysts. The next big step, however, will be to integrate the water oxidation half reaction with the carbon dioxide reduction step in an artificial leaf type system.
"The efficiency, speed and size of our cobalt oxide nanocrystal clusters are comparable to Photosystem II," said Frei. "When you factor in the abundance of cobalt oxide, the stability of the nanoclusters under use, the modest overpotential and mild pH and temperature conditions, we believe we have a promising catalytic component for developing a viable integrated solar fuel conversion system. This is the next important challenge in the field of artificial photosynthesis for fuel production."
Frei and Jiao have reported the results of their study in the journal Angewandte Chemie, in a paper entitled: "Nanostructured Cobalt Oxide Clusters in Mesoporous Silica as Efficient Oxygen-Evolving Catalysts." This research was performed through the Helios Solar Energy Research Center (Helios SERC), a scientific program at Berkeley Lab under the direction of Paul Alivisatos, which is aimed at developing fuels from sunlight. Frei serves as deputy director of Helios SERC.






Comments