Researchers studying a critical stage of pregnancy – implantation of the embryo in the uterus – have found a protein that is vital to the growth of new blood vessels that sustain the embryo. Without this protein, which is produced in higher quantities in the presence of estrogen, the embryo is unlikely to survive.
This is the first study to detail the mechanism by which the steroid hormone estrogen spurs cell differentiation and blood-vessel growth in the uterus during pregnancy, the researchers report.
The findings, from researchers at the University of Illinois, Emory University, Baylor College of Medicine and New York University, appear in the journal Development.
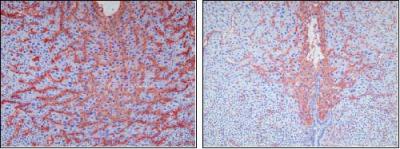
Connexin 43 (Cx43) belongs to a family of proteins that form junctions between cells that regulate the flow of ions and small signaling molecules from cell to cell. At the time of embryo implantation, this gap junction protein is essential to the rapid growth of new blood vessels needed to support the development of the embryo and allow it to implant in the uterine wall, the researchers discovered.
The researchers chose to study Cx43 after analyzing genes that are activated in the presence of estrogen in uterine cells. They found that Cx43 was prominent among the genes whose expression was increased in cells after exposure to estrogen.
University of Illinois veterinary biosciences doctoral student Mary Laws studied the role of Cx43 in pregnant mice and in human endometrial cells. By deleting the Cx43 gene in the uterus immediately after pregnancy in mice, a technique developed by researchers at Baylor, Laws was able to reliably prevent implantation of the embryo in the uterus.
In human endometrial cells (provided by co-author Robert Taylor of Emory University), Cx43 enhanced the differentiation of cells that make up the stromal tissue of the uterus. These cells produce factors that promote the growth of new blood vessels.
One of the factors secreted by the endometrial cells, vascular endothelial growth factor (VEGF), is essential to angiogenesis. In the absence of Cx43, Laws found, the cells failed to differentiate or to produce enough VEGF to spur blood vessel growth.
"The formation of these new blood vessels is extremely critical for embryonic growth at this stage of pregnancy, when the embryo has begun to invade into the uterine tissue, but has yet to make a connection to the placenta where it ultimately gets its nutrients," said Illinois veterinary biosciences professor Indrani Bagchi, corresponding author on the study. "I think this is the first animal model that shows that disruption of one particular molecule or gene leads to a defect in uterine angiogenesis."
The findings have important implications for early pregnancy loss and female infertility, she said.
"A fundamental aspect of female reproductive biology is how these hormones signal in uterine tissue in order to support the pregnancy," said molecular and integrative physiology professor Milan Bagchi, an author on the study. "One of our major goals is to identify the genes that are regulated by estrogen and progesterone precisely at the time when the embryo implants in the uterine wall."
"Connexin 43 has been shown to be in the uterus in many animal systems – cows and pigs and rodents and humans," Laws said. "But this is the first time that it's been shown to be critical for pregnancy."
This research was sponsored by the recently established Center for Research in Reproduction and Infertility, which is funded by the National Institute of Child Health and Human Development. Based at the U. of I., the center also draws expertise from Emory University Medical School and Baylor College of Medicine.






Comments