A team of Penn State University researchers is the first to demonstrate that lipid molecules in cell membranes participate in mammals' reactions to allergens in a living cell. The finding will help scientists better understand how allergy symptoms are triggered, and could contribute to the creation of improved drugs to treat them.
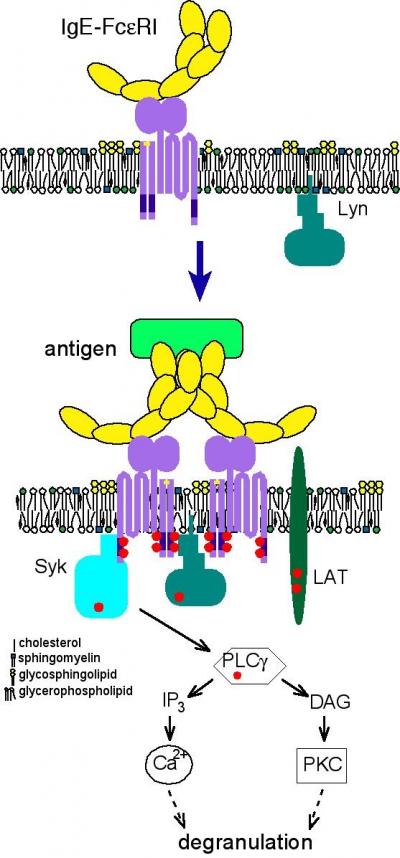
The team studied clusters of cholesterol-rich lipid molecules that they believe serve as platforms for the receptors that receive antibodies, the proteins that protect the body from allergens. In this case, the team examined IgE antibodies, which upon binding to their receptors initiate a cell's release of histamine--the substance that causes the unpleasant, but beneficial, mucous production, congestion, and itchiness associated with allergies.
"This research is basically the molecular foundation for why many people sneeze in the spring," said Ahmed Heikal, an associate professor in the Department of Bioengineering and a leader of the project.
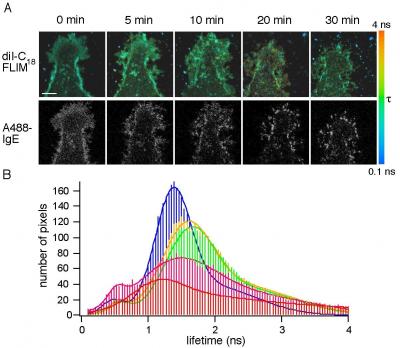
While the idea that lipid clusters--also known as lipid domains--are involved in the allergic response is not new, the Penn State team is the first to document this connection in a living cell under physiological conditions. "No one has observed the domains in action because they are too small and too transient--held together by very weak molecular interactions--to be viewed with a light microscope," said Erin Sheets, a Penn State assistant professor of chemistry who also is a leader of the project. "To overcome this challenge," added Heikal, "we used a combination of imaging and spectroscopy techniques that we are developing in our laboratories.
In their experiment, the researchers first labeled the cell membrane and IgE antibodies with two different fluorescent tags. Next, they introduced an allergen and watched as it bound to receptors on the cell membrane, thus initiating an allergic response.
But to demonstrate that this activity was taking place within the lipid domain, the researchers had to take advantage of a property of fluorescence, called fluorescence lifetime, in which molecules are excited with very short laser pulses. The length of time a molecule remains in its excited state before emitting a photon--the fluorescence lifetime--provides unique information about the fluorescently-labeled molecule's environment and its chemical structure. For example, a particular molecule might relax to its lowest-energy state quickly or slowly depending on whether it is exposed to a solvent.
"We previously showed that our fluorescently-labeled membrane probe has a longer lifetime within a cholesterol-rich lipid domain," said Sheets. "Here we show that changes in this lifetime follow the changes that occur during the first steps in the allergic response process. Our results also show that lipid domains in the cell membrane associate with IgE antibodies and their receptors in the initial stages of an allergic reaction."
In the future, Sheets and Heikal plan to apply the team's discoveries to a project involving aging. During the aging process, T cells, which protect the body from foreign substances like viruses and cancer cells, can lose their ability to signal effectively. Sheets and Heikal plan to use these fluorescence-lifetime imaging tools to examine the structure and integrity of T-cell membranes with a goal of determining why they lose their knack for signalling and how this problem can be corrected.
"We want to compare the effectiveness of signaling in young T cells, which clear out debris quickly, to old T cells, which are not as efficient," said Sheets. "I think it will be a pretty cool application of our technique."
Other Penn State scientists who contributed to this research include Angel Davey and Keith Krise, both Ph.D. students in the Department of Chemistry. The work was funded by Penn State, the National Science Foundation, the Commonwealth of Pennsylvania, the American Chemical Society, and the National Institutes of Health.
Article: Angel M. Davey, Keith M. Krise, Erin D. Sheets, and Ahmed A. Heikal, Molecular Perspective of Antigen-mediated Mast Cell Signaling, J. Biol. Chem., Vol. 283, Issue 11, 7117-7127, March 14, 2008 doi:10.1074/jbc.M708879200





Comments