A new paper speculates the root cause might be the microbiome. The authors argue that in certain micro-environments like the gut, if the gut microbiome produces high levels of metabolites, like those found in certain bacteria and foods that claim "antioxidant" properties like black tea and hot cocoa, then it may act aid mutated genes and accelerate the growth of bowel cancers. The new study is only in mice, and mice are not little people, so the findings are only exploratory and should not be used to make any changes to your lifestyle, no matter what supplement companies start selling.
Starting in the 1970s, antioxidants became a health fad. Since our cells engage in reduction and oxidation as part of metabolism, the belief was that too much oxidation would cause misfires inside cells. An entire supplement and diet fad built up around the idea that mitochondria had somehow survived a billion years but drinking more Earl Grey tea would invade its protective membrane and do something good. The problem with the diet and supplement fad was the same as with microbiome claims now; with so many variables, changing things in carpet bombing fashion by high doses of vitamin E could do more harm than good. Most likely they did nothing at all.
The new paper suggests levels of gut bacteria are the issue: small intestines contain few, whereas colons contain a lot. They then draw a line from that to the current belief in the microbiome being the secret sauce to many diseases, which set off the probiotic fad.
The study
Researchers introduced mutated p53 proteins into the gut of Csnk1a1-defcient and ApcMin/+ mice. The small intestine reacted by converting the mutated p53 cancer driver back to normal p53 and better at suppressing cancer growth than healthy p53 proteins. However, when mutated p53 was introduced into the colon, they stayed truer to their cancer mission and promoted the cancerous spread. In the small bowel they totally switched course and attacked the cancerous cells, whereas in the colon they promoted the cancerous growth.
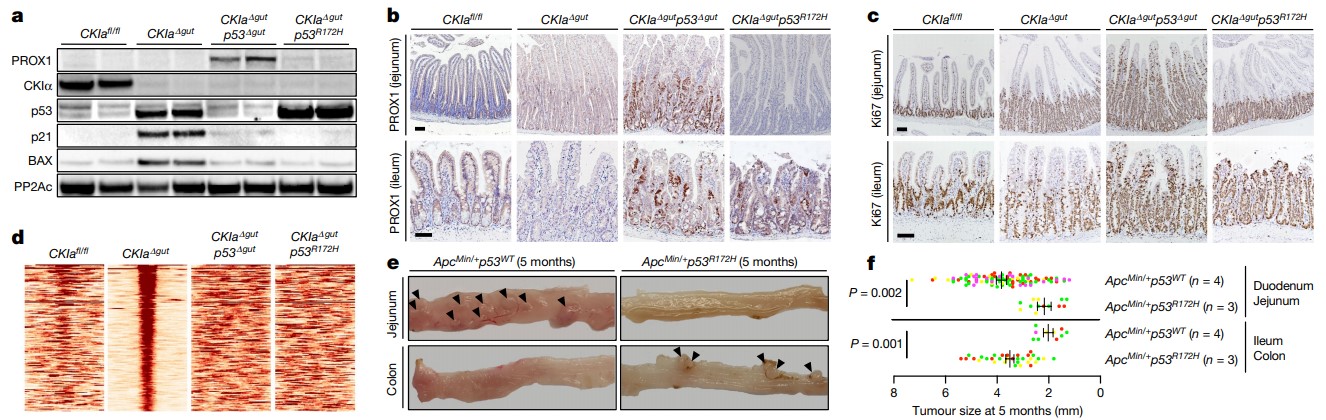
Mutant p53 counteracts dysplasia and tumorigenesis in the proximal gut without regaining wild-type transcriptional activity. a, Immunoblot of mouse jejunal enterocytes. PP2Ac, loading control. For gel source data, see Supplementary Fig. 1. b, c, IHC of the invasion and dysplasia marker PROX1 (b) and the proliferation marker Ki67 (c) in mouse jejunum and ileum. Scale bars, 100 μm. d, p53 ChIP–seq-derived heat maps from mouse jejunal enterocytes. e, Representative images of different segments of the mouse bowel. Arrowheads indicate visible tumours. f, Tumour size (each mouse is colour-coded). Overall average of the mean values for each mouse ± s.e.m (n, number of mice), one-sided Student’s t-test. Representative data from six (a–c) or three (e) independent experiments.
To further test their belief that gut flora was a major factor as to why mutated p53 were acting as tumor blockers in the small bowel but tumor accelerants in the colon, the scientists administered antibiotics to kill off the colon's gut flora. Once they did, the mutated p53 was not able to go on its cancer spree.
What's in this flora that makes colon cancer spread so quickly? They speculate that gut flora produces metabolites, aka "antioxidants", which are found in high concentrations in foods such as black tea, hot chocolate, nuts and berries. When the scientists fed mice an antioxidant-rich diet, their gut flora accelerated p53's cancer-driver mode.






Comments