Researchers have long been concerned that could happen in the HIV-1 virus, which has been mitigated thanks to safer sex practices and modern science but still affects 38 million people worldwide.
A new, highly virulent HIV strain has erupted in the Netherlands and individuals infected with the new “VB variant” (for virulent subtype B) showed significant differences before antiretroviral treatment compared with individuals infected with other HIV variants:
Individuals with the VB variant had a viral load (the level of the virus in the blood) between 3.5 and 5.5 times higher.
In addition, the rate of CD4 cell decline (the hallmark of immune system damage by HIV) occurred twice as fast in individuals with the VB variant, placing them at risk of developing AIDS much more rapidly.
Individuals with the VB variant also showed an increased risk of transmitting the virus to others.
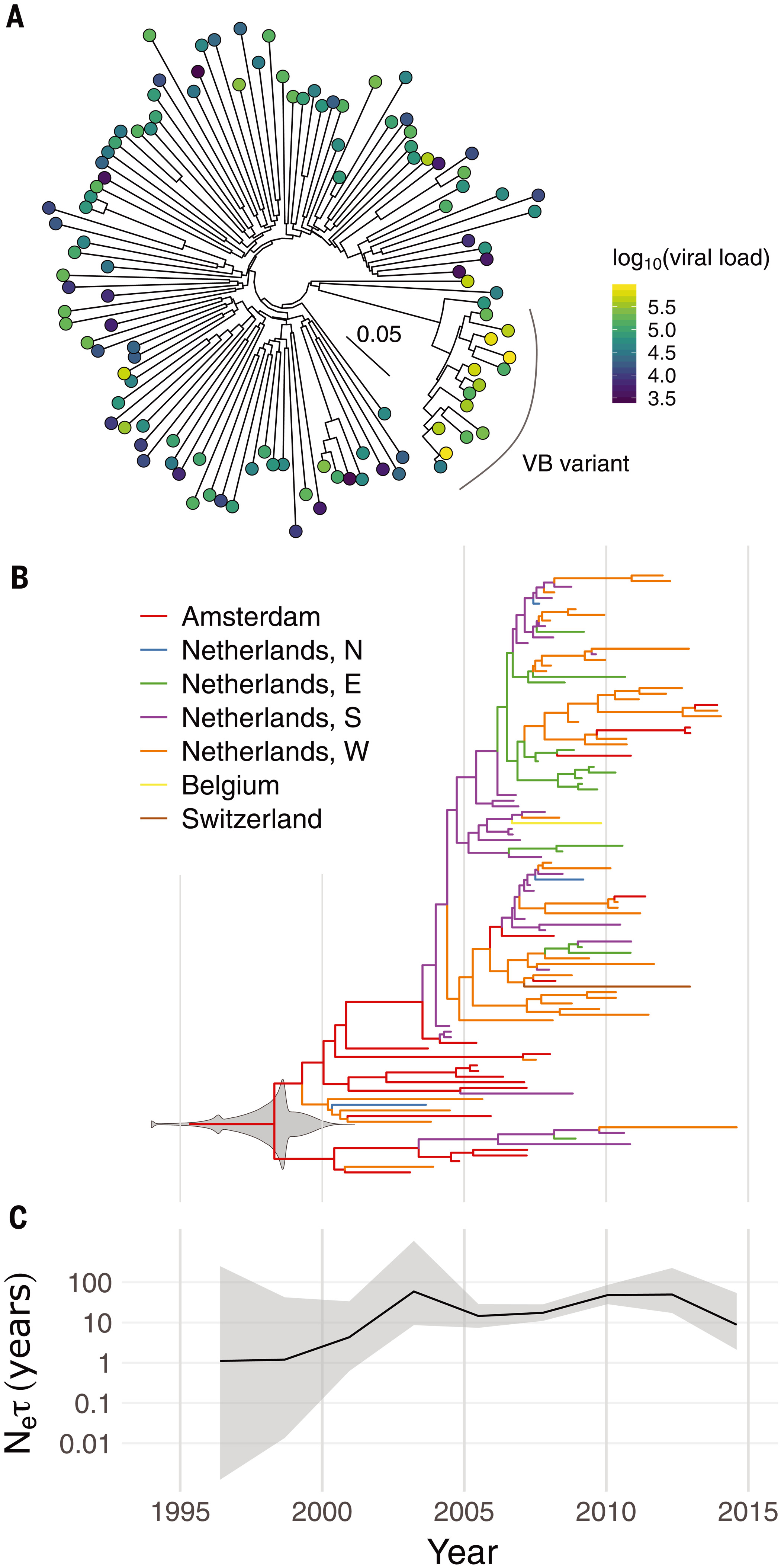
Phylogenetic and phylodynamic analysis of the VB variant.(A) Whole-genome maximum-likelihood phylogeny of 15 VB-variant sequences and 100 background subtype-B sequences. The color of each circle indicates the individual’s viral load in log10 copies per milliliter. The inset scale bar shows the branch length scale in units of substitutions per site. (B) Dated maximum-clade-credibility tree for 107 partial pol gene sequences from the VB variant. Colors indicate geographical regions (N, E, S, and W: north, east, south, and west), which are known for the tips of the tree but are otherwise inferred by ancestral state reconstruction. The gray violin plot superimposed on the root node shows the posterior density for its date (i.e., the TMRCA); 1994 contains overflow to earlier dates for clarity. (C) Effective population size (Ne) (scaled by the coalescent generation time τ) over time with 95% credibility intervals, with the same time axis as in (B).
The VB variant was first identified in 17 HIV positive individuals from the BEEHIVE project, an ongoing study which collects samples from across Europe and Uganda. Since 15 of these people came from the Netherlands, the researchers then analyzed data from a cohort of over 6,700 HIV positive individuals in the Netherlands. This identified an additional 92 individuals with the variant, from all regions of the Netherlands, bringing the total to 109.
By analyzing the patterns of genetic variation among the samples, the researchers estimate that the VB variant first arose during the late 1980s and 1990s in the Netherlands. It spread more quickly than other HIV variants during the 2000s, but its spread has been declining since around 2010. The research team believe that the VB variant arose in spite of widespread treatment in the Netherlands, not because of it, since effective treatment can suppress transmission.
The individuals with the VB variant showed typical characteristics for people living with HIV in the Netherlands, including age, sex, and suspected mode of transmission. This indicates that the increased transmissibility of the VB variant is due to a property of the virus itself, rather than a characteristic of people with the virus.
The good news is that even though the VB variant causes a more rapid decline in immune system strength, once treatment starts individuals with the VB variant had similar immune system recovery and survival to individuals with other HIV variants. Early detection remains key.
Next, researchers want to understand the mechanism that causes the VB variant to be more transmissible and damaging to the immune system. The VB variant is characterized by many mutations spread throughout the genome, meaning that a single genetic cause cannot be identified at this stage.






Comments