The molecule, called Fibroblast growth factor (FGF) works like a 'switch', inhibiting and activating the genes for different tissues in order to obtain a balanced heart. But the researchers also found that manipulation of its levels allowed them to control the tissue(s) that developed, in what might be the first step towards treatments able to grow heart or blood vessels tissues according to the patient's specific needs. The study in the journal Development is on zebrafish, but FGF is known to affect the human heart as well, including the growth of new blood vessels.
One other interesting aspect of the study – because it might explain a key steppingstone of
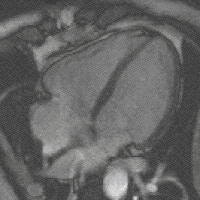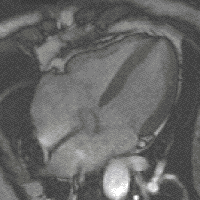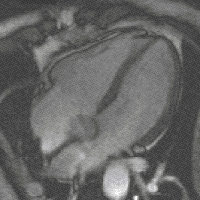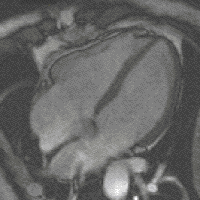 evolution - is the fact that high levels of FGF create more cardiac muscle leading to bigger hearts. And this - says Filipa Simões, one of the first authors of the study – could have been the mechanism behind the appearance of 4-chambered hearts (zebrafish has only 2), which, more energetically efficient, allowed warmed-blooded animals to emerge changing Earth’s landscape forever.
evolution - is the fact that high levels of FGF create more cardiac muscle leading to bigger hearts. And this - says Filipa Simões, one of the first authors of the study – could have been the mechanism behind the appearance of 4-chambered hearts (zebrafish has only 2), which, more energetically efficient, allowed warmed-blooded animals to emerge changing Earth’s landscape forever. (Four- chamber heart , image by magnetic resonance)
CVD are the number one cause of death in the world, killing as much as 17 millions of people
every year. These are conditions that normally involve the blocking of blood vessels with one of its major complications being the infarcts of the myocardium - when the blocking of the heart vessels leads to death of the cardiac muscle (the myocardium) due to the lack of oxygen. Even if the patient survives, the rigid scar tissue that is formed overworks the remaining muscle increasing susceptibility to new episodes. To make things worse, the human heart regeneration is incredibly low , in fact so low that it was only proved 3 years ago when radioactive isotopes, released into the atmosphere during the Cold War above-ground nuclear testing, were found in the heart of people born before the tests (showing that those cells were new).
This lack of self repair , a CVD epidemic that is now also spreading to developing countries and no effective heart regeneration treatments available with stem cells still to live up to their hype, has made research into new approaches to repair the heart crucial. And in this sense, a population of progenitor cells called haemangioblasts found in embryos of animals with 2-chambered hearts is particularly promising.
Progenitor cells are similar to stem cells, just more differentiated, for example contrary to stem cells they tend to be able to develop only one type of tissue . But not haemangioblasts, which not only form two - blood and endothelial - but apparently have the potential to develop a third - the myocardium. Additionally, they are believed to be the evolutionary precursors of the 2nd heart field - an area in the embryo of 4 -chambered heart animals responsible for most of its cardiac muscle and believed to be the added "extra bit" that during evolution led to animals with bigger hearts and more chambers ( culminating in the 4 chambers of mammals and birds). Interestingly the 2nd heart field is also home to another population of progenitor cells, which is able to develop several heart tissues (like haemangioblasts?). This genetic/evolutionary link, together with haemangioblasts’ “multi-tissue capacity” , suggested that their study could give us important clues on our own heart and also on mechanisms to regenerate it.
To understand better these cells and their link to the heart Filipa Simões, Tessa Peterkin and Roger Patient used (2-chambered heart) zebrafish embryos and looked into the effects of FGF on the development of haemangioblasts and the region right next to it, the heart field. FGF was interesting because it was known to be important for heart development, including our own, although its exact effects and the mechanisms behind them were still very unclear.
So normally in zebrafish the heart field area develops the myocardium, while haemangioblasts form endothelial tissue (that is part of both the heart and the blood vessels) and blood cells. Simões and Peterkin started by inhibiting FGF in the embryos and to their surprise, with FGF levels reduced, the heart field lost its cardiac markers (markers are genes/molecules linked to a specific development) and developed instead those typical of endothelial/blood cells. Haemangioblasts also showed an increase in their markers suggesting that FGF had been inhibiting the “endothelial/blood program” in both places. And this was not a transitory as the change was traced as far as differentiated cells.
Since there was no alteration in the total of number of cells in the two areas, and the FGF effects occurred simultaneous on both of them, the more logical explanation was that the cells in the heart field were changing their fate from myocardium to blood/endothelial tissues. Change of fate in adjacent embryonic areas has been known to occur if the two development programs antagonise each other and haemangioblasts development (which is the first to be activated in the embryo) was already known to inhibit that of the heart field.
So they did the opposite experiment and introduced a molecule that promotes cardiac development (and is induced by FGF)in high quantities in the embryos to see now haemangioblasts “losing their path” and developing cardiac markers, with the heart field increasing their levels as well. So FGF not only inhibited the haemangioblasts program but also activated that of the myocardium.
In conclusion, FGF seems to switch off the expression of blood and endothelial genes enough to keep their development under control while at the same time promotes the growth of cardiac tissue assuring, in this way, a balanced heart. But if FGF levels change, the results become very different – increased FGF leads to expansion of the myocardium as the endothelial/blood program is totally inhibited and cardiac genes are activated, while reduced quantities of FGF, no longer able to stop the endothelial/blood growth, see these tissues inhibiting cardiac development and taking over both embryonic regions.
A first implication of these results is that they give support to the idea that haemangioblasts are in fact the evolutionary precursors of the mammals’ 2nd heart field by providing a mechanism for this to occur. As FGF levels increase, cardiac genes are activated both in the heart field and in the haemangioblasts, expanding the myocardium and forming larger hearts with more chambers. Interestingly, amphibian, found in the evolution tree between fish and mammals, have 3–chambered hearts and a residual population of haemangioblasts in what could be seen as an intermediate stage.
But can this work help to regenerate our own heart?
First it will be necessary to show that FGF has a similar effect on humans. We already know that this molecule promotes the growth of new blood vessels on the human heart, and with haemangioblasts having an evolutionary (genetic) link with our 2nd heart field other similarities will no doubt exist. It would be interesting, for example, to look at FGF effects on that 2nd heart field’s population that seems similar to haemangioblasts. But therapies that can deliver specific tissues according to the patient’s need - instead of using, for example, stem cells, which are now seen as the big therapeutic bet – can be, not only more efficient, as stem cells can sometimes have problems producing enough tissue, but would also avoid dangers such as teratomas – a potential malign tumour linked to stem cells.
It could be interesting as well to investigate a possible link between FGF and congenital heart defects - which are still the most common problem in newborns and the number one cause of death in the 1st year of life - as many seem to derive from problems in the definition of the heart tissues.
There is something else of note in this new study - the fact that it gives support to the theory that all heart tissues come from a common precursor cell (which so far had only be seen on blood cells) since both the heart field and haemangioblasts show a capacity to form multiple heart tissues (myocardium and endothelium). This is particularly interesting because it would mean a change of paradigm and probably the need to re-evaluate our approach to biologic therapies for the heart.
Citation: Filipa Costa Simões, Tessa Peterkin, and Roger Patient, 'Fgf differentially controls cross-antagonism between cardiac and haemangioblast regulators', Development 2011 August 138, 3235-3245 doi: 10.1242/dev.059634





Comments