A fallacy is a pattern of logical reasoning which appears on its surface to be a pattern of sound reasoning. The fallacy of the average is based on the false notion that the effect of a thing averaged out on a large scale is equivalent to an effect of the same thing on a small scale.
A drop of rain falling anywhere in the Pacific is self-evidently insignificant as a matter of scale.
But what if that single drop of rain falls into the mains supply circuit of a radio?
If I have a box of screws and wish to know their average length, I need only take some random sample and measure each screw in the sample. Using sound statistical methods, I can determine the likelihood of anyone picking a screw at random of a specific length range.
It may be that the average screw is 15.5 mm long. It seems to be self-evident that if I pick three screws at random, then they will measure, end-to-end 46.5 mm. In reality, that is a most unlikely outcome if the box is of mixed sizes.
Again, if I have a box of 1,000 10 mm cubes, 70% of which are of copper and 30% of zinc, then I most definitely do not have, on average, a box of 5.84803548mm3 brass cubes.
A fallacy of the averagely overheated human
The fallacy: compared with all the salt in all the oceans, the amount of salt in human sweat is so trivial that it can be ignored for all practical purposes at the scale of human society and climate.
This fallacy of averages tells us that human sweat is insignificant compared with natural sources of atmospheric salt. However, global averages take no account of clustering. What is the effect of the accumulated sweat of a large human population in an urban heat island?

Boulder, Colorado at night.
The reddish glow from the city lights of Boulder, Colo., is the result in part of the light being scattered by haze particles. UW scientists have discovered unexpected chemistry involving the pollutants that make up the haze.
The smell of salt air, a mile high and 900 miles inland.
Vince Stricherz March 10, 2010
"It's there. We know it's there. But we don't have a good handle on where that chloride comes from," said Joel Thornton, a University of Washington associate professor of atmospheric sciences and lead author of a paper documenting the findings, published March 11 in Nature.
http://uwnews.org/article.asp?articleID=56206
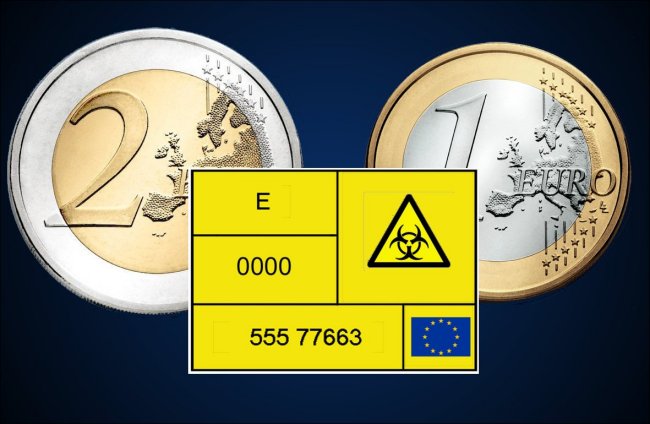
How many euros does a breach of European regulations cost?
The European regulation 94/27/CE of June 30th, 1994 requires that for items in direct contact with the skin, the amount of nickel release shall be lower than or equal to 0.5 mg/cm2 week.
1 euro and 2 euro coins have been shown to release from 240 to 320 times more nickel than regulations allow, due to human sweat, according to a September 2002 Nature article.
-----
[1] - credit to Henry Cox for suggesting that human sweat in an urban heat island might account for at least some of the atmospheric sodium chloride discovered by the researchers at Boulder, Colorado.





Comments