WASHINGTON D.C. Feb. 18, 2014 -- Calico cats, renowned and beloved for their funky orange and black patchwork or "tortoiseshell" fur, can thank X chromosome inactivation or "silencing" for their unique look.
A team of University of California San Francisco (UCSF) researchers is striving to unlock the mystery of how one X chromosome can be rendered nearly completely inactive. They will present their latest results at the 58th Annual Biophysical Society Meeting, which takes place Feb. 15-19, 2014, in San Francisco, Calif.
The cells of female mammals contain two copies of the X chromosome, one each from mom and dad, but because cells only need one active X, the other one is "turned off". Calico cats have an orange fur color gene on one of their X chromosomes and a black fur color gene on the other, so that the random silencing of one of the X's in each cell creates their distinctive patchwork coats. But while such manifestations of X chromosome inactivation have long been observed, researchers are still unclear of exactly how a cell silences a chromosome.
The UCSF researchers approached this mystery by first finding a way to image the X chromosome in its natural position within an intact cell. "A cell's nucleus contains the genetic code, its DNA. But while the structure of the DNA was determined more than 50 years ago, and we're rapidly determining the position of specific genes on chromosomes, no one had visualized the DNA within an intact nucleus -- an unfixed, hydrated whole cell," explained Elizabeth Smith. "We decided to try."
Smith is a postdoctoral fellow working in Carolyn Larabell's lab in the Anatomy Department at UCSF. Larabell is the director of the National Center for X-Ray Tomography, which is where the instrument development is taking place.
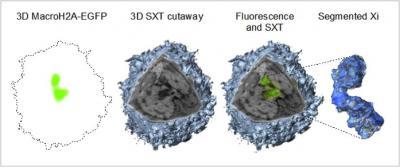
This image shows the first ever high-resolution structure of an inactive X chromosome (far right) its native state by combining a new pair of emerging imaging technologies.
(Photo Credit: E.A.Smith/UCSF)
The work could eventually help researchers better understand how many different kinds of genes can be turned on or off without altering the underlying DNA sequence. "The inactivation of one out of two X chromosomes in females is an enormously important epigenetic process," said Smith. "Uncovering how only one X chromosome is inactivated will help explain the whole process of epigenetic control, meaning the way changes in gene activity can be inherited without changing the DNA code. It can help answer other questions such as if and how traits like obesity can be passed down through generations."
To visualize the DNA within an intact nucleus, Smith and colleagues turned to a novel imaging technology, soft x-ray tomography. "We obtained high-resolution, 3-dimensional views of the intact nucleus and, by using a prototype cryo fluorescence microscope along with the x-ray microscope, we were able to identify one specific chromosome, the inactive X chromosome of female cells," Smith said.
The team imaged and analyzed the inactive X chromosome in a number of different cells and was surprised by the wide variation in the structural organization adopted by the chromosome. "We were able to show a remarkable substructural organization of this chromosome, which consists of three distinct domains of differing amounts of chromatin," said Smith.
To obtain their results the researchers developed a new "correlated imaging" technique. This new form of microscopy has a wide range of possible future applications, particularly to identify the position of specific molecules within the densely packed environment of the nucleus. "With new fluorescent probes, we can start identifying the position of specific genes in context -- inside the tangled network of DNA within the intact nucleus," Smith explained.
While this work is still at the basic research stage, it shows potential to have a significant impact on understanding, diagnosing, and treating X-chromosome-linked diseases in the future.






Comments