Scientists have identified a gene, Lhx1, that regulates sleep and wake rhythms.
The discovery of the role Lhx1 provides scientists with a potential therapeutic target to help night-shift workers or jet lagged travelers adjust to time differences more quickly. T
Every cell in the body has a "clock" – an abundance of proteins that dip or rise rhythmically over approximately 24 hours. The master clock responsible for establishing these cyclic circadian rhythms and keeping all the body's cells in sync is the suprachiasmatic nucleus (SCN), a small, densely packed region of about 20,000 neurons housed in the brain's hypothalamus.
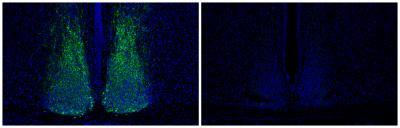
A peptide responsible for cell communication in the brain, Vip (green) is reduced in the brains of mice that have little or no Lhx1 (right). Credit: Salk Institute for Biological Studies
More so than in other areas of the brain, the SCN's neurons are in close and constant communication with one another. This close interaction, combined with exposure to light and darkness through vision circuits, keeps this master clock in sync and allows people to stay on essentially the same schedule every day. The tight coupling of these cells also helps make them collectively resistant to change. Exposure to light resets less than half of the SCN cells, resulting in long periods of jet lag.
In the new study, researchers disrupted the light-dark cycles in mice and compared changes in the expression of thousands of genes in the SCN with other mouse tissues. They identified 213 gene expression changes that were unique to the SCN and narrowed in on 13 of these that coded for molecules that turn on and off other genes. Of those, only one was suppressed in response to light: Lhx1.
"It's possible that the severity of many dementias comes from sleep disturbances," says Satchidananda Panda, a
Salk Institute for Biological Studies
associate professor who led the research team. "If we can restore normal sleep, we can address half of the problem.
"No one had ever imagined that Lhx1 might be so intricately involved in SCN function," says Shubhroz Gill, a postdoctoral researcher and co-first author of the paper. Lhx1 is known for its role in neural development: it's so important, that mice without the gene do not survive. But this is the first time it has been identified as a master regulator of light-dark cycle genes.
By recording electrical activity in the SCN of animals with reduced amounts of the Lhx1 protein, the researchers saw that the SCN neurons weren't in sync with one another, despite appearing rhythmic individually.
"It was all about communication–the neurons were not talking to each other without this molecule," says Ludovic Mure, a postdoctoral researcher and an author on the paper. A next step in the work will be to understand exactly how Lhx1 affects the expression of genes that creates this synchronicity.
Studying a mouse version of jet lag–an 8-hour shift in their day-night cycle–the scientists found that those with little or no Lhx1 readjusted much faster to the shift than normal mice. This suggests that because these neurons are less in sync with one another, they are more easily able to shift to a new schedule, though it is difficult for them to maintain that schedule, Panda says.
These mice also exhibited reduced activity of certain genes, including one that creates vasoactive intestinal peptide or Vip, a molecule that has important roles in development and as a hormone in the intestine and blood. In the brain, Vip affects cell communication, but nobody had known that Lhx1 regulated it until now, Panda says. Interestingly, the team also found that adding Vip restored cell synchrony in the SCN.
"This approach helped us to close that knowledge gap and show that Vip is a very important protein, at least for SCN," Panda says. "It can compensate for the loss of Lhx1."
On the other hand, cutting back on Vip could be another way to treat jet lag. Vip could be an even easier drug target compared with Lhx1 because Vip is secreted from cells rather than inside cells, Panda says. "If we find a drug that will block the Vip receptor or somehow break down Vip, then maybe that will help us reset the clock much faster," he adds.
The new results take the group a step closer to their goal of creating cell regenerative therapies that restore the SCN and ameliorate sleep problems. The scientists have made their gene expression data available through a searchable web interface at http://scn.salk.edu, giving other researchers a handy way to explore the effect of light and dark in genes in the SCN and other tissues.






Comments