So the kids fell asleep late last night after finally crashing from their Halloween sugar high. There are smashed pumpkins out on the street, toilet paper in the trees, and still 3 to 5 pounds of candy remaining per child. The pumpkins and toilet paper can be cleaned up… but what do you do with all that candy? You can’t possibly eat it all, right?
Well go ahead and give it a your best shot. But a week from now when it’s still around and you can’t stand the sight of the stuff, you’ll be happy to know that science once again has a solution.
What could be a better combination than candy and science? (Well, except for chocolate and peanut butter, of course.) Take a handful of scientific principles, mix them liberally with a pile of leftover candy… and Voila! Yummy, sugary, scientific goodness!
So grab a chocolate bar, and enjoy. Candy isn’t just for eating anymore.
10. Marvel in the engineering brilliance and efficiency of the shape of M&M’s.
M&M’s are one of my all-time favorite candies. Their colorful, compact, and they travel well. They’re fantastic alone: but also pretty damn good sprinkled over ice cream, baked in cookies, or folded into Rice Krispy treats.
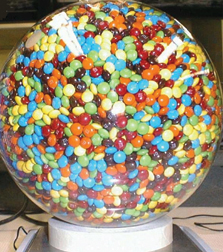
But M&M’s have developed a whole new group of fans in the science community, and it has nothing to do with how they taste. It seems that M&M’s are a marvel of packing efficiency, and are actually leading to the next generation of design for heat shields and reduced-porosity glass with exceptional transparency.
It seems that the simple act of pouring a bag of M&M’s into a bowl illustrates the propensity of squashed or stretched versions of spheres snuggle together more tightly than randomly packed spheres do. Really, it’s been proven… with bags and bags of M&M’s, and hours of study from talented and dedicated grad students.
According to Sidney R. Nagel of the University of Chicago. "This work is really beautiful. It enhances our understanding of one of the outstanding questions in science - namely, how densely various types of objects can pack together.”
Well said, Sidney. However, don’t be surprised if the University Alumni don’t get a bit upset to discover that part of their generous donations potentially went towards buying candy for grad students to play around with.
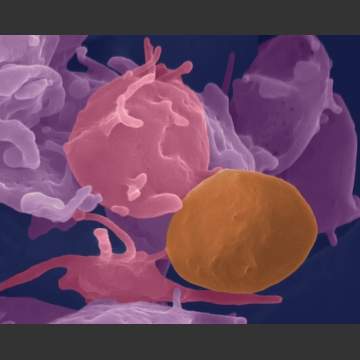
I suppose it makes sense though, right? Before M&M/Mars got into the act, the human body recognized the efficiency of this particular shape when creating platelets. The component of the blood that must be the MOST efficient at packing together densely to create a blood clot are surprisingly shaped similarly to our snuggly-shaped M&M’s.
Don’t believe me, or those geniuses at the University of Chicago? Grab some of those leftover bags of M&M’s; and their plumper, rounder, fruit-flavored cousins, the Skittles; and compare how many of each you can fit into the same space. I bet you’ll find that you’ll be able to squeeze in several more of the sleeker and streamlined M&M’s than you will of the pudgier Skittles. And good news, you’ve used up some of your excess inventory of candy-coated treats.
9. Add some Spark to the Dark with Wint-O-Green Lifesavers.

This little trick has been around since I was a little girl. (Yes, way back in the olden days). Stand in front of the mirror in a dark bathroom, and crunch up some Wint-O-Green Lifesavers with your teeth. You’ll be tickled to see a little lightning storm erupt in your mouth. (While you’re in there, make sure you suppress the urge to turn around three times and call out “Bloody Mary”. Even if you are a big fan of the hangover cure… according to legend as well as last week’s episode of “Ghost Whisperer,” only bad things can happen if you mutter the name when alone in a dark bathroom.)
Actually, all hard sugar-based candies emit some degree of light when you bite them, but most of the time, that light is very faint. This effect is called triboluminescence, which is similar to the electrical charge build-up that produces lightning, only much less grand. Triboluminescence is the emission of light resulting from something being smashed or torn. When you rip a piece of tape off the roll, it will produce a slight glow for the same reason.
Triboluminescence occurs when molecules, in this case crystalline sugars, are crushed, forcing some electrons out of their atomic fields. These free electrons bump into nitrogen molecules in the air. When they collide, the electrons impart energy to the nitrogen molecules, causing them to vibrate. In this excited state, and in order to get rid of the excess energy, these nitrogen molecules emit light – mostly ultraviolet (nonvisible) light, but they do emit a small amount of visible light as well. This is why all hard, sugary candies will produce a faint glow when cracked.

But when you bite into a Wint-O-Green Life Saver, a much greater amount of visible light can be seen. This brighter light is produced by the wintergreen flavoring. Methyl salicylate, or oil of wintergreen, is fluorescent, meaning it absorbs light of a shorter wavelength and then emits it as light of a longer wavelength. Ultraviolet light has a shorter wavelength than visible light. So when a Wint-O-Green Life Saver is crushed between your teeth, the methyl salicylate molecules absorb the ultraviolet, shorter wavelength light produced by the excited nitrogen, and re-emit it as light of the visible spectrum, specifically as blue light -- thus the blue sparks that jump out of your mouth when you crunch on a Wint-O-Green Life Saver.
Fun! And minty-fresh breath to boot!
8. Monster Marshmallows
Anyone that has made Smores in the microwave has witnessed this fun little phenomenon. Throw a marshmallow in, turn the oven on high, and see a real life attack of “The Blob”.
Luckily for us, Peeps aren’t just for Easter any more. Now a days, you can find Pumpkin and Ghost shaped sugar-coated marshmallow treats, and in fact you may have discovered some in your Trick-or-Treat bag this morning. And as pretty and sparkly as they may be, I’ve never enjoyed the taste of them much. So to me a much more appealing alternative is to microwave the suckers, and see the ghosts and pumpkins grow and expand like creepy, blobby monsters.
Marshmallows are mostly sugar and water wrapped around a bunch of air bubbles. When you cook marshmallows in your microwave oven, several things happen at once. The microwave makes the water molecules vibrate very quickly—which makes the water heat up. The hot water warms the sugar, which softens a little. The hot water also warms the air bubbles.
When you warm air in a closed container, the gas molecules move around faster and push harder against the walls of the container. As the air in the bubbles warms up, the air molecules bounce around faster and faster and push harder against the bubble walls. Since the sugar walls are warm and soft, the bubbles expand, and the marshmallow puffs up. If it puffs up too much, some air bubbles burst, and the marshmallow deflates like a popped balloon.
A bubbly, blobby ghost/pumpkin shaped mess. What fun!
7. Kitchen Chemistry: Candy Cookies
Cooking is a science of sorts… kind of like chemistry where you mix things, add heat, and see what results. Lucky for us, the results are much yummier in the kitchen as opposed to the chemistry lab. So here’s some “kitchen chemistry” you can apply to any number of leftover treats, and end up with something much tastier than the original ingredients alone.
The experiment is simple. Stuff chocolate chip cookie dough into some mini muffin tins, squish in your choice of chocolaty treat, and throw it into the oven and bake. The result is these delicious little muffin-shaped cookies with a chocolate treasure baked inside. This works great with Hershey’s Kisses, mini peanut-butter cups, Rolos… even Milk Duds. Pretty much any smallish chocolate treat that you can imagine would blend well with a warm chocolate chip cookie.
Here’s the recipe. Feel free to try with a variety of candies… and let us know how they turn out! I’m always looking for a new chocolate/cookie combination.
1 package refrigerated chocolate chip cookie dough
24 Hershey's kisses (or mini peanut butter cups, or rolos, etc...)
1. Preheat oven to 350°F. Separate dough into 24 equal size squares.
2. Lightly spray mini-muffin tin with vegetable cooking spray. Place one piece of dough into each cup. Squish a single piece of candy into the center of each square of cookie dough, so dough pushes up and around candy slightly.
3. Bake 10-12 minutes. Let cookies cool in pan 15 minutes; remove to plate or cooling rack.
Yes... that simple. And that good.
Another helpful note, these cookies travel really well. So if you are ever thinking of sending of some fresh baked cookies in a care package, or like to ship cookies to family at Christmas… these cookies are excellent candidates. Their compact “muffin” shape is very hardy, and resists crumbling throughout the trials of travel. And since the chocolate is trapped safely inside a baked cookie shell, these cookies won’t melt even if shipped in the middle of July. Trust me, you’ll be buying MORE candy, just to make these cookies.
6. M&M Duels
As I mentioned earlier, M&Ms are one of my favorite candies. I’m clearly not alone in this adoration, as there are many folks out there that have sung their praises. And interestingly enough, there seems to be as many traditions on how to eat M&Ms as there are fans that eat them.
The following approach is my favorite. The author is unknown (edit: Tim Shaw, aka
Timantec, tells us he wrote this on his xanga account Feb 11, 2003 but the site is now gone), but I have to admit I’ve held my share of duels myself. But I could never describe the process better than this…
Whenever I get a package of plain M&Ms, I make it my duty to continue the strength and robustness of the candy as a species. To this end, I hold M&M duels.
Taking two candies between my thumb and forefinger, I apply pressure, squeezing them together until one of them cracks and splinters. That is the "loser," and I eat the inferior one immediately. The winner gets to go another round.
I have found that, in general, the brown and red M&Ms are tougher, and the newer blue ones are genetically inferior. I have hypothesized that the blue M&Ms as a race cannot survive long in the intense theatre of competition that is the modern candy and snack-food world.
Occasionally I will get a mutation, a candy that is misshapen, or pointier, or flatter than the rest. Almost invariably this proves to be a weakness, but on very rare occasions it gives the candy extra strength. In this way, the species continues to adapt to its environment.
When I reach the end of the pack, I am left with one M&M, the strongest of the herd. Since it would make no sense to eat this one as well, I pack it neatly in an envelope and send it to M&M Mars, A Division of Mars, Inc., Hackettstown, NJ 17840-1503 U.S.A., along with a 3x5 card reading, "Please use this M&M for breeding purposes."
This week they wrote back to thank me, and sent me a coupon for a free 1/2 pound bag of plain M&Ms. I consider this "grant money." I have set aside the weekend for a grand tournament. From a field of hundreds, we will discover the True Champion.
There can be only one…
5. Transform Candy into Christmas Ornaments
There’s nothing I admire more than the polished art of “re-gifting”. So what a great discovery to find a way to take leftover Halloween candy, add a sprinkle of science, and end up with something you can ship to the family as personalized Christmas gifts. Will they be happy to receive it? I don’t know… that’s more than science can determine. But it will help you get rid of some of your excess candy, and appear crafty and thoughtful at the same time.
So let’s do some transforming. Nothing as exotic as those alien/car/robots that entertained us in the movie theaters this summer… but a transformation none the less. Collect some of your overabundance of hard candy, lollypops, and lifesavers. Then shape them and heat them to create sweet stained glass Christmas ornaments.
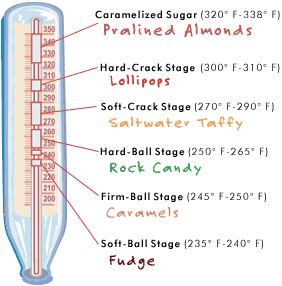
Different types of candy are formed a different heats. You soft chewy kinds form at a lower heat; while the harder crystallized candies require a much higher temperature. So to “transform” the hard candy used for this pretty little experiment, you have to reintroduce these candy pieces to the same temperature that was used to create them in the first place.
You will need:
Heavy metal cookie cutters (large copper cutters and they work great)
Vegetable spray
Life Saver or other hard candies
Aluminum foil
Cookie sheet
Straw
Narrow shiny ribbon
Preheat oven to 350°. Line your cookie sheet with aluminum foil and spray the cookie cutters and aluminum foil with vegetable cooking spray. Fill the inside of the cookie cutters with a single layer of candy using as many as will fit. Bake 5 to 7 minutes until candies are melted.
Remove from oven and allow candy to cool about 2 minutes. Make a hole in each with a straw to thread ribbon through for hanging then continue cooling until the cutters can be handled. Very gently pull cutters away from ornament. Thread with ribbon, and they will be ready to hang on the Christmas tree.
Ta-Dah! Sweet and pretty ornaments, made from the candy friends and neighbors handed out to you last night. Go ahead, wrap them up and give them to the neighbors at Christmas, and see if they recognize their own treats.
4. Re-enact the Scientific Experiment that supposedly Killed “Mikey”
It was a great day in 1975 when Pop Rocks first hit the candy shelves. There were like nothing we had seen before, and soon kids all across the nation were standing in front of one another with their mouths open, enjoying the sights and sounds of the popping, sizzling candy that burst into action as soon as they touched your tongue.
But it wasn’t long until tragedy struck, and the story started circulating around the school grounds of how “Mikey” from the Life cereal commercial died a horrible death as a result of a fatal overdose of Pop Rocks and carbonated soda. As the story goes, the chipmunk-cheeked youngster ate 6 bags of Pop Rocks, and then proceeded to drink a six-pack of cola. The two substances fatally combined in his stomach and exploded, killing him on the spot.
Well it made for a great story, and one we were happy to repeat to all our friends. Of course as it often happens, there wasn’t an ounce of truth to it. The actor that played Mikey not only survived his childhood unexploded, but currently works as an advertising-account manager for a radio station in New York. And the fatal combination of Pop Rocks and soda? Well, it’s much less fatal than the rumors would have you believe. But General Mills, the company that produced the candy, had their hands full in the late 70’s disputing the exploding kid stories. They took out full-page ads in 45 major publications, wrote some 50,000 letters to school principals around the country, and sent the candy’s inventor on the road to explain to everyone that Pop Rocks generate less gas than half a can of soda, and ingesting them could induce nothing worse in the human body than a hearty, non-life-threatening belch. Despite all these measures, the rumors abound even to this day.
So what’s the science behind Pop Rocks?
Here's the basic idea. Hard candy is made from sugar, corn syrup, water and flavoring. You heat the ingredients together and boil the mixture to drive off all of the water. Then you let the temperature rise. What you are left with is a pure sugar syrup at about 300 degrees F (150 degrees C). When it cools, you have hard candy.
To make Pop Rocks, the hot sugar mixture is allowed to mix with carbon dioxide gas at about 600 pounds per square inch (psi). The carbon dioxide gas forms tiny, 600-psi bubbles in the candy. Once it cools, you release the pressure and the candy shatters, but the pieces still contain the high-pressure bubbles (look at a piece with a magnifying glass to see the bubbles).
When you put the candy in your mouth, it melts (just like hard candy) and releases the bubbles with a loud POP! What you are hearing and feeling is the 600-psi carbon dioxide gas being released from each bubble.
Pop Rocks are back on the shelves today, after a brief re-naming and re-marketing campaign in the 80’s. If you happen to find any in the trick-or-treat pile, and you’re the type to throw caution to the wind… go ahead and down a packet or two with a soda chaser and see what truth there is to the rumors. Please understand however I’m not encouraging this. If you have to find out for yourself, do so at your own risk. I don’t want to be responsible for any exploding science enthusiasts, or even any bad stomach aches.
3. Determine the Number of Licks to get to the Center of a Tootsie Pop
I’m sorry for all the reminiscing, but remember those cartoon commercials from the 70’s with the wise owl in the tree answering the greatest mystery in the universe: how many licks does it take to get to the center of a Tootsie Pop? Of course if you do remember, you’ll recall that the greedy owl was no help because he bit into the Tootsie Pop after lick three.
Well, the question still exists today. And if you have an overabundance of Tootsie Pops sitting around your house today, there’s not time like the present to finally find the answer.
But luckily for you, science has improved the process a bit from those good old days. Instead of straining your tongue to determine the number, you can let a nifty bit of technology discover the answer for you. (And no, it’s not 42)
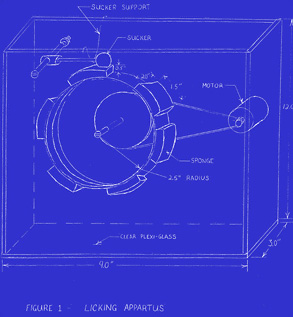
A group of engineering students from Purdue University designed a licking machine, modeled after a human tongue. After several trials, they discovered the machine took an average of 364 licks to get to the center of a Tootsie Pop.
However, when they tried the same licking test on 20 volunteers they found that the average licks to the center were 252 licks. Why the difference? I can only assume it was the temperature differential. Their nifty machine may have been able to duplicate the licking motion, but not the temperature of a 98.6 degree human tongue. This is only a hypothesis on my part of course. Feel free to create your own experiment with a “heated” licking-sponge machine, and see if I’m correct. Let me know what you find out. I’ll be right here, searching for Nestle “100 Grand” bars in my daughters trick-or-treat bag.
2. Use Extra Chocolate Bars as a Viable Alternative Energy Source.
I ran across this little scientific discovery when researching my article of “ Top Ten Scientific Reasons that Chocolate is the World's Most Perfect Food." It seems that researchers at the UK’s University of Birmingham fed Escherichia coli bacteria a feast of waste caramel and nougat from a chocolate candy bar. The bacteria subsequently burped out hydrogen gas, which was harnessed via a fuel cell to power an electric fan.
That’s right, feed your excess Milky Way bars to some nasty E-coli bacteria, and produce a green energy source to run your household appliances! I’m sure there are some extra steps in there somewhere… but I want to do right by the environment, am a big fan of chocolate, and trying to find a use for all this leftover Halloween candy. So this discovery is a trifecta in my book.
1. Create a Mentos geyser
Okay, this it at the top of my list because it’s just about the most exciting thing you can do with candy outside of actually eating it. Who among us didn’t want to create our version of the erupting volcano science experiment, except without that boring part of constructing the volcano? Really, it was the exploding part that got all of our attention anyway, right? So let’s get right to it. And if you are unlucky enough to live in a town where neighbors actually gave out Mentos for Halloween, this is a much more appealing use for them than reenacting those goofy commercials.
This experiment will generate an explosion similar to the one rumored in the great Pop Rocks/Soda debate… but without the debate. This will literally put on quite a show, but is something you definitely don’t want to duplicate in your stomach. All you need is a roll of Mentos, a 2-Liter of Diet Coke, and lots of open space that can get hosed down after the activity is over.

1. Carefully open the bottle of soda. Position the bottle on the ground so that it will not tip over.
2. Unwrap the whole roll of Mentos. The goal is to drop all of the Mentos into the bottle of soda at the same time (which is trickier than it looks). One method for doing this is to roll a piece of paper into a tube just big enough to hold the loose Mentos. You'll want to be able to position the tube directly over the mouth of the bottle so that all of the candies drop into the bottle at the same time.
3. Don't drop them into the bottle until you warn nearby spectators to stand back. Then, drop the Mentos in and get out of the way!
The result will be an eruption of soda that has been known to create a blast that crests at over 18 feet.
So what’s the science behind this amazing display? Well, the jury is still out on exactly why these two food items react so violently. But many believe it is a physical reaction, not a chemical one.
Water molecules strongly attract each other, linking together to form a tight mesh around each bubble of carbon dioxide gas in the soda. In order to form a new bubble, or even to expand a bubble that has already formed, water molecules must push away from each other. It takes extra energy to break this "surface tension." In other words, water "resists" the expansion of bubbles in the soda.
When you drop the Mentos into the soda, the gelatin and gum arabic from the dissolving candy break the surface tension. This disrupts the water mesh, so that it takes less work to expand and form new bubbles. Each Mentos candy has thousands of tiny pits all over the surface. These tiny pits are called nucleation sites - perfect places for carbon dioxide bubbles to form. As soon as the Mentos hit the soda, bubbles form all over the surface of the candy.
Couple this with the fact that the Mentos candies are heavy and sink to the bottom of the bottle and you've got a double-whammy. When all this gas is released, it literally pushes all of the liquid up and out of the bottle in an incredible soda blast.
Regardless of the scientific reasons behind it, it’s an amazing phenomenon to witness. If you don’t feel like blasting soda all over your house, you can take a look at some video of others that have successfully performed the experiment. All the fun, but none of the mess!
So there you have it. If you get tired of enjoying your horde of Halloween candy the old fashioned way, you’re now armed with any number of entertaining methods to put all of those sugar-filled snacks to use. Feel free to add your own ideas to the list. I’m sure there is no shortage of households out there that will be grateful for additional ways to shrink the candy pile now sitting in a bowl on top of their kitchen counter.







Comments