For
quite a few years, one of the most popular chemicals for scientific inquiry has
been bisphenol
A (BPA).
Scientists around the world have been conducting a diverse array of
studies aimed at understanding whether BPA poses a risk to human health.
Based on the weight of evidence from these
many studies, the U.S. Food and Drug Administration (FDA) recently answered the
question “Is BPA safe?”
with a simple and unambiguous answer - “Yes.”
By far, the majority of the studies conducted on BPA provide information on potential hazards, which are intrinsic properties of the chemical. Since risk describes the probability that exposure to a hazard will cause harm, information is also needed on human exposure to BPA.
Considering that BPA is a commonly usedchemical, primarilyas a raw material to make polycarbonate plastic and epoxy resins, it is not surprising to find that people are exposed to BPA. Of critical importance, though, is the magnitude of exposure. If there is no exposure, there can be no risk and, conversely, only when exposure is sufficiently high would risks potentially be present.
An
increasingly accepted way to assess human exposure is through biomonitoring studies, which
measure the level of a chemical in biological samples such as urine or
blood. For BPA, analyzing urine is most
appropriate since BPA is converted in the body to a biologically inactive
metabolite and excreted in urine within hours of exposure. Urine biomonitoring thus measures short-term
exposure to BPA over the last day or so.
When applied to a representative group of people, urine biomonitoring can
provide a reasonable measure of average exposure to BPA across a population.
Recent U.S. and Canadian Data
Two
recently published large-scale urine biomonitoring studies provide up-to-date
exposure data for the U.S. population (age 6 years and above) and Canadian pregnant
women, respectively. The U.S. data is
from a long-standing program run by the Centers for Disease Control and
Prevention (CDC) and known as the National Health and Nutrition Examination
Survey (NHANES).
The most recent data,
from samples collected in 2011-2012, is contained in the fifth set of biennial
data over a 10-year period, which allows temporal exposure trends to be
assessed.
The Canadian data is from the Maternal-Infant Research on Environmental Chemicals (MIREC) study, which is sponsored by Health Canada and other Canadian government agencies. The study provides exposure data for a large population ofwomenin the first trimester of pregnancy, with samples collected in the 2008-2011 timeframe. The MIREC data is particularly valuable since pregnant women might generally be considered to be one of the most vulnerable subpopulations.
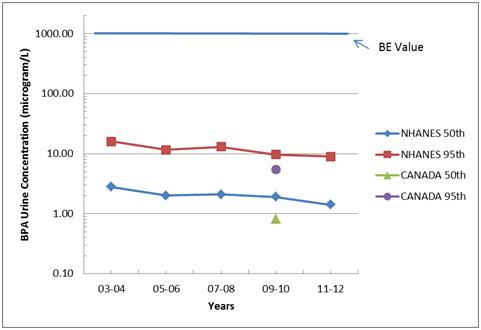
The median and 95th percentile urinary BPA concentrations from both new studies are shown in the figure below. Also shown for assessment of temporal trends are NHANES data from four earlier biennial studies in the 2003-2010 timeframe.

A quick way to understand this type of data in a safety context is to compare the measured urinary concentration values with a Biomonitoring Equivalent (BE) value. A BE is defined as “the concentration or range of concentrations of chemical in a biological medium (blood, urine, or other medium) that is consistent with an existing health-based exposure guidance value such as a reference dose (RfD) or tolerable daily intake (TDI).” Essentially a BE value is an estimated safe limit.
Several years ago, Health Canada sponsored a project to calculate a BE value for BPA in urine. Based on Health Canada’s TDI for BPA, the BE value was calculated as 1,000 micrograms/L, which is shown as a bar across the top of the figure.
Casual
inspection of the figure reveals that even 95th percentile urine
concentrations of BPA are approximately 2 orders of magnitude below the BE
value. Typical human exposure, in the
range of the median values, is hundreds of times below the BE value and Health
Canada’s safe intake limit. Although BE
values are primarily useful as screening tools, the large margin of safety
between the BE value and the recent biomonitoring data clearly indicates that
the U.S.population and pregnant women in Canada are not at risk from real-lifeexposures
to BPA.
Accordingly, in its announcement
of the MIREC data, Health Canada stated “Based on the overall weight of evidence, Health
Canada continues to conclude that dietary exposure to BPA through food
packaging is not expected to pose a health risk to the general population,
including newborns and young children.”
A
second interesting feature in the figure is the apparent downward trend in
exposure to BPA from 2003-2004 to 2011-2012.
The trend might best be described as suggestive since the decrease in
urine concentrations is small and we cannot be certain whether the decrease is
due to subtle changes in the NHANES program (e.g., analytical methodology,
participant selection, urine sampling) or is due to decreased exposure in the
US population.
Although biomonitoring data are a very useful measure of exposure
levels, the data provide no information on sources of
exposure, which makes it difficult to determine if the sources have changed
over time.
In
spite of the clear statements of safety from government agencies such as FDA
and Health Canada, it would nevertheless not be surprising if some limited deselection
of BPA has taken place in certain markets.
In that case, it would be of particular interest to know what is being
used to replace BPA.
That’s a
fascinating subject, but one that will discussed another day in the near
future.





Comments