With nuclear power trapped in committee meetings and biofuels exposed as a bad idea, the search for another carbon-neutral alternative is ongoing.
While the sun gives us enough energy in an hour to meet all human needs for a year, solar technologies are an ideal solution that needs better technology - just like it has been for 50 years.
Conversion of solar energy into electrochemical energy on a massive-scale first needs to show proof-of-concept on the micro-scale. An artificial version of photosynthesis is regarded as one of the most promising of solar technologies because for two billion years nature has employed photosynthesis to oxidize water into molecular oxygen.
Berkeley Lab researchers, working at the Joint Center for Artificial Photosynthesis (JCAP), have developed the first fully integrated microfluidic test-bed for evaluating and optimizing solar-driven electrochemical energy conversion systems. This test-bed system has already been used to study schemes for photovoltaic electrolysis of water, and can be readily adapted to study proposed artificial photosynthesis and fuel cell technologies. The JCAP mission is to develop an artificial version of photosynthesis through specialized membranes made from nano-engineered materials that can do what nature does only much more efficiently and for the purpose of producing storable fuels such as hydrogen or hydrocarbons (gasoline, diesel, etc.).
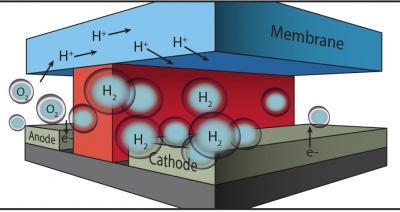
In this microfluidic test-bed, a chemically inert wall (red) separates anode from cathode and the channels in which O2 and H2 are generated by splitting water. Protons (H+) are conducted from one channel to the other via a membrane cap (Nafion®) that also prevents the intermixing of the O2 and H2 product streams. Image courtesy of Miguel Modestino, Berkeley Lab and JCAP
Miguel Modestino, lead author of the paper, says, "In our experimental realization of the design, a series of 19
parallel channels were fabricated in each device, with a total active area of eight square millimeters. As the microfabricated chips are relatively easy to make, we can readily change
dimensions and materials to optimize performance."
"We've demonstrated a microfluidic electrolyzer for water splitting in which all functional components can be easily exchanged and tailored for optimization," says Joel Ager, a staff scientist with Berkeley Lab's Materials Sciences Division. "This allows us to test on a small scale strategies that can be applied to large scale systems. The operating principles of artificial photosynthetic systems are similar to redox flow batteries and fuel cells in that charge-carriers need to be transported to electrodes, reactants need to be fed to catalytic centers, products need to be extracted, and ionic transport both from the electrolyte to catalytic centers and across channels needs to occur. While there have been a number of artificial photosynthesis demonstrations that have achieved attractive solar to hydrogen conversion efficiencies, relatively few have included all of the operating principles, especially the chemical isolation of the cathode and anode."
The microfluidic test-bed allows for different anode and cathode materials to be integrated and electrically accessed independently through macroscopic contacts patterned in the outside of the microfabricated chip. The transport of charge-carriers occurs through an ion conducting polymer membrane, and electrolysis products can be evolved and collected in separated streams. This general design provides selective catalysis at the cathode and anode, minimization of cross-over losses, and managed transport of the reactants.
Virtually any photoelectrochemical component, including those made of earth-abundant elements, can be incorporated into the test-bed.
The paper "Integrated microfluidic test-bed for energy conversion devices" is in the journal Physical Chemistry Chemical Physics (PCCP). Co-authors are Rachel Segalman, Miguel Modestino, Camilo Diaz-Botia, Sophia Haussener and Rafael Gomez-Sjoberg.






Comments