One of your earliest science memories in school is learning that, during photosynthesis, plants take in carbon dioxide sunshine and produce oxygen. Later we all learned that in lakes and oceans a similar process happens due to cyanobacteria.
What has remained unknown is exactly how that happens.
Oxygen formation in photosynthesis occurs in a reaction sequence that is completed within one thousandth of a second, so it's not surprising that it has been so difficult to prove experimentally how precisely a catalyst consisting of four manganese ions and one calcium ion (Mn4Ca cluster) performs this reaction sequence in photosystem II.
Almost all molecular details we presently 'know' about the last critical steps are based on calculations. A team of researchers have now explored two different ways for obtaining experimental insight into the mechanism of oxygen formation.
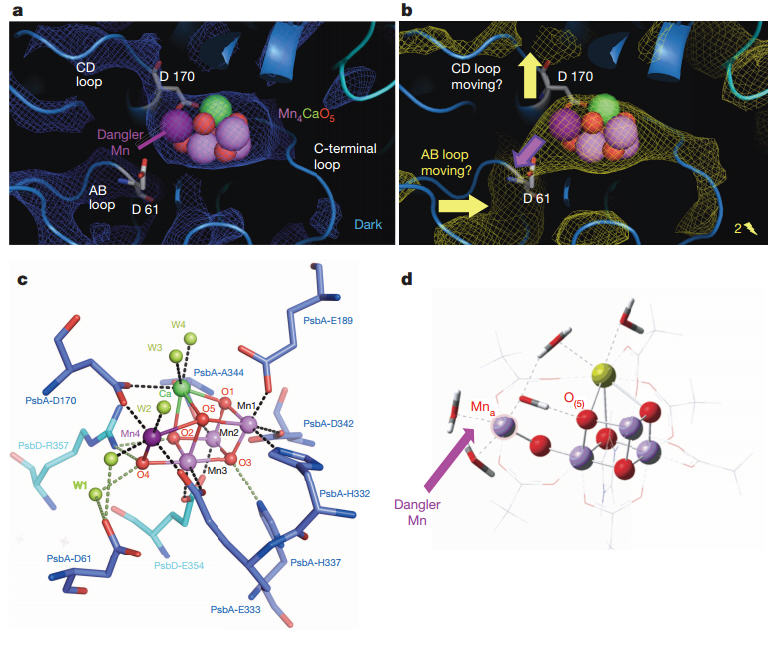
The OEC simulated annealed omit maps. See DOI: 10.1038/nature 13453
"The new knowledge will help improving present day synthetic catalysts for water oxidation, which are key components for building artificial leaf devices for the direct storage of solar energy in fuels like hydrogen, ethanol or methanol," says Johannes Messinger, Professor in Biological Chemistry and leader of the Artificial photosynthesis research group at Umeå University.
In the first study, the collaborators used a X-ray free electron laser, Linac Coherent Light Source (Menlo Park, USA), that produces ultra-short high-intensity x-ray pulses (10−15 of a second) to perform simultaneous x-ray crystallography and x-ray emission spectroscopy on suspensions of micrometer sized photosystem II crystals.
"With this technique we studied the same reaction sequence and we obtained 'snap shots' of the structure of the atoms for the different states of the cluster, including the short lived state investigated in the first study."
The data show that no large scale structural changes (> 0.5·10−10 m) occur in the Mn4Ca cluster and the rest of the photosystem II complex during oxygen formation. The simultaneously collected X-ray emission data confirm that the "arresting" of the two bound water molecules, as observed in the mass spectrometric experiments, is not due to a change in the charge (oxidation state) of the manganese ions of the Mn4Ca cluster, nor to the formation of a first bond between the oxygen atoms of the two water molecules.
"The study suggests that small structural changes occur together with the proton release, but we still need to further improve the resolution of our data to see exactly what happens."
In another study, performed in collaboration with two French researchers, they slowed down the reaction sequence more than 40-times by exchanging the calcium of the cluster against strontium, and a nearby chloride ion against an iodide ion.
"We could show that in the last short-lived intermediate state before oxygen formation, the two water molecules are 'arrested', meaning that they are more than 1000-times more tightly bound to the Mn4Ca cluster than in all earlier states of the reaction. This stabilization is thought to be caused by a previously reported loss of a proton and to reflect a highly ordered arrangement that is required for the fast and energy efficient formation of oxygen from water."
That result was obtained using oxygen isotopic labeling and time-resolved membrane inlet mass spectrometry.






Comments