#3 in a series

Stephen Hales (1677-1761) was a clergyman who devoted much of his time to scientific pursuits, especially in conducting experiments in plant physiology.
His most important work, Vegetable Staticks (published in 1727), was in plant physiology.
The dedication is to George Prince of Wales, afterwards George III. ... the real interest of the dedication is its clear statement of his views on the nutrition of plants. He asserts that plants obtain nourishment, not only from the earth, " but also more sublimed and exalted food from the air, that wonderful fluid, which is of such importance to the life of Vegetables and Animals," etc. We shall see that his later statement is not so definite, and it is well to rescue this downright assertion from oblivion.Hales concluded from his experiments that not only did plants draw sustenance from the air, but that since light enters leaf surfaces it may play a role in plant growth and development.
Stephen Hales, by Francis Darwin, Makers of British Botany, 1913.
"The change of bodies into light, and of light into bodies, is very conformable to the course of nature, which seems dehghted with transformations." It is a problem for the antiquary to determine, whether or no Swift took from Newton the idea of bottling and recapturing sunshine as practised by the philosopher of Lagado. He could hardly have got it from Hales, since Gulliver's Travels was published in 1726, before Vegetable Staticks.
Sir Francis Darwin
Rustic sounds and other studies in literature and natural history, 1917, page 136
"Quest. 30. Are not gross Bodies and Light convertible into one another, and may not Bodies receive much of their Activity from the Particles of Light which enter their Composition? For all fix'd Bodies being heated emit Light so long as they continue sufficiently hot, and Light mutually stops in Bodies as often as its Rays strike upon their Parts, ...Hales' experiments with living things led him to conclude, correctly, that fresh air is conducive to good health. He was a pioneer in the mechanical ventilation of enclosed spaces. His discovery that air could be chemically modified led to the invention of CO2 absorption devices later used in submarines and space capsules.
Isaac Newton in Opticks 4th edition 1717
It has happened again and again in the history of discovery that some of the most important advances in a particular science have been made by persons not engaged in the professional pursuit of that subject.
Hales died in 1761, thirteen years before oxygen was discovered. It is interesting to note that Hales had the most definite conceptions as regards this necessity for oxygen in ventilation, without knowing what it was that sustained life, and without knowing, in anything like its fulness, the meaning and importance of Joseph Black's discovery that animals exhaled carbon dioxide from their lungs. Black's discovery was published in 1754, some seven years, indeed, before Hales died; but it is certain that Hales was not indebted to Black; on the contrary, it is not as widely known as it might be that Black was profoundly indebted to Hales. Black wrote :
I was partly led to these experiments by some observations by Dr. Hales, in whieh he says that breathing through diaphragms of cloth dipped in alkaline solution made the air last longer for the purposes of life.
Carbon dioxide was discovered under the name of gas sylvestre by the Belgian chemist J. B. van Helmont (1577 to 1644) about the year 1640.
Mayow died in 1679 ; and in England nothing was done as regards respiration or ventilation until Hales arose to rediscover much that Lower and Mayow had known well.
D. Fraser Harris, Stephen Hales the Pioneer in the Hygiene of Ventilation, 1916
Stephen Hales invented the pneumatic trough, a device for collecting gases in an inverted container over water. The device was later used by such pioneers as Joseph priestley.
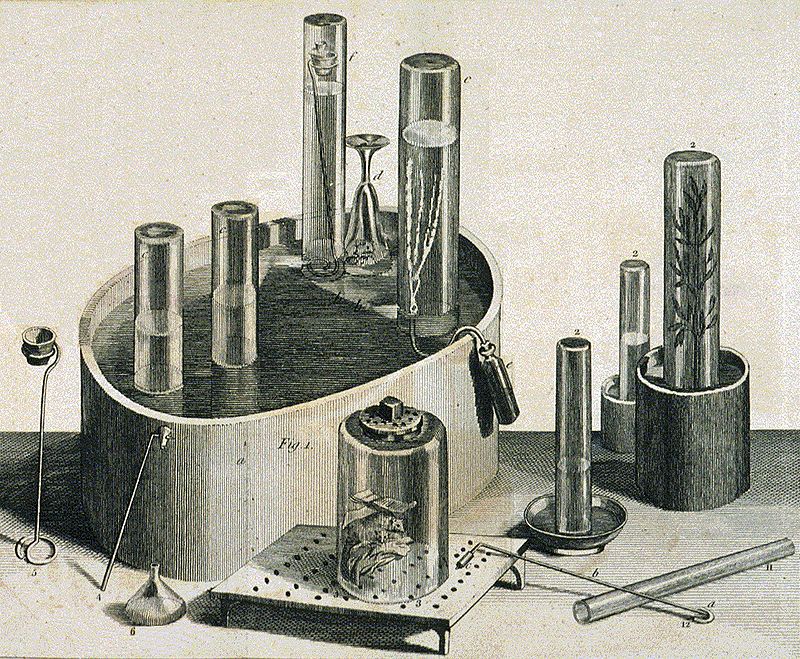
Pneumatic trough, and other equipment, used by Joseph Priestley
Joseph Priestley (1733-1804) - Frontispiece of
Experiments and Observations on Different Kinds of Air
Public domain image, courtesy Wikipedia
It is interesting to note that Hales showed that, put in simple modern terms, a gas could combine with a solid to create a new solid and that a gas could evolve from a solid. This moved chemistry and physics a long way from previous theories about the classic forms of matter: air, earth, fire and water.
Sir William Ramsay wrote an excellent history of the study of our atmosphere. The text below is an abstract from the part of Ramsay's book concerning Stephen Hales. Previous parts published here at science20.com were about John Mayow and Robert Boyle. The text below, courtesy archive.org. is error-checked for typos.
Hales' chief work is entitled " Statical Essays, containing Vegetable Staticks ; or an account of Statical Experiments on the Sap in Vegetables, being an Essay towards a Natural History of Vegetation : of use to those who are curious in the Culture and Improvement of Gardening, etc. : Also, a specimen of an attempt to analyse the air by a great Variety of Chymiostatical Experiments, which were read at several meetings before the Royal Society. By Stephen Hales, D.D., F.R.S., Rector of Farringdon, Hampshire, and Minister of Teddington, Middlesex."
In his " Introduction" Hales reveals his method of research. The determination of weight and volume was at that date especially necessary ; for want of numerical data the experimental researches of the time were of a somewhat vague character, and it often happened that the conclusions drawn from them were incorrect. ...
From experiments on the rise of sap in plants, many of them very ingenious and well adapted to secure their end, and which are still regarded by botanists as classic, Hales noticed that a quantity of air was inspired by plants. In order to ascertain the composition and amount of this air, the process of distillation was resorted to ; for Hales remarks : " That elasticity is no immutable property of air is further evident from these experiments ; because it were impossible for such great quantities of it to be confined in the substances of animals and vegetables, in an elastick state, without rending their constituent parts with a vast explosion" (Preface, p. viii). Hence, concluding that the air absorbed by plants and animals could be recovered by their distillation, Hales proceeded to distil a great number of substances of animal and vegetable origin, such as hogs' blood, tallow, a fallow-deer's horn, oyster-shell, oak, wheat, peas, amber, tobacco, camphor, aniseed oil, honey, bees'-wax, sugar, Newcastle coal, earth, chalk, pyrites, a mixture of salt and boneash, of nitre and bone-ash, tartar, compound aquafortis, and a number of other substances. He collected the " air " in each case over water, and gave numerical data to show what proportion the air bore by weight to the substance from which it had been obtained. He even tried to compare the weight of ordinary air with that of air from distilled tartar ; but his experiment led to no positive conclusion, because of the crudeness of his appliances. The compressibility or " elasticity " of the air from tartar, however, was found to be identical with that of common air.
Hales does not appear to have made any special experiments on the properties of his various airs, by trying whether they supported combustion, whether they were themselves combustible, etc. We see from this list that he had under his hands mixtures of hydrocarbons, carbon dioxide, probably sulphur dioxide, hydrochloric acid and ammonia (both, however, dissolving in water as they were formed), oxides of nitrogen, possibly chlorine, and, as minium or red-lead was one of the substances he tried, oxygen in a more or less pure state. It must be remembered that in all cases the gas obtained was mixed with the air originally present in the retort. He next proceeded to produce "air" by the fermentation of grain, of raisins, and of other fruits ; this "air" obviously was carbon dioxide more or less pure.
...
The next series of experiments related to the generation of " air " by the action of acids on metals. Aqua-regia and gold, aqua-regia and antimony, aquafortis and iron, dilute oil-of-vitriol and iron, yielded gases which contracted on standing in contact with water. This, in the case of the oxides of nitrogen, is to be ascribed to their reacting with the oxygen of the air accidentally present in the receiver ; but in the last case Hales noticed that the gas absorbed in cold weather was re-evolved on rise of temperature, as one would expect with hydrogen.
These experiments led him to investigate the action of certain mixtures on ordinary air. Thus a mixture of spirits of hartshorn (or ammonia) with iron filings absorbed 1 1/2 cubic inches of air, and one with copper filings twice as much. Further, a mixture of iron filings and brimstone absorbed in two days no less than 19 cubic inches of air.
But it is disappointing to find that, in spite of all the experimental facts which Hales accumulated, he was unable to make use of them. The prejudice in favour of the unity and identity of all these " airs " was too great for him to overcome. True, he sometimes theorises a little, as for example when he remarks (p. 285) : " If fire was a particular kind of body inherent in sulphur (i.e. combustible matter of all kinds), as Mr. Homberg, Mr. Lemery, and some others imagine, then such sulphureous bodies, when ignited, should rarefy and dilute all the circumambient air ; whereas it is found by many of the preceding experiments, that acid sulphureous fuel constantly attracts and condenses a considerable part of the circumambient elastick air : an argument that there is no fire endued with peculiar properties inherent in sulphur ; and also that the heat of fire consists principally in the brisk vibrating action and re-action between the elastick repelling air and the strongly attracting acid sulphur, which sulphur in its Analysis is found to contain an inflammable oil, an acid salt, a very fix'd earth, and a little metal."
Enough has now been said to give a fair idea of Stephen Hales' researches. It will suffice if his conclusions be stated in his own words (p. 314) :
" Thus, upon the whole, we see that air abounds in animal, vegetable, and mineral substances ; in all which it bears a considerable part; if all the parts of matter were only endued with a strongly attracting power, whole nature would then immediately become one unactive cohering lump ; wherefore it was absolutely necessary, in order to the actuating and enlivening this vast mass of attracting matter, that there should be everywhere intermix'd with it a due proportion of strongly repelling elastick particles, which might enliven the whole mass, by the incessant action between them and the attracting particles ; and since these elastick particles are continually in great abundance reduced by the power of the strong attracters, from an elastick to a fixt state, it was therefore necessary that these particles should be endued with a property of resuming their elastick state, whenever they were disengaged from that mass in which they were fixt, that thereby this beautiful frame of things might be maintained in a continual round of the production and dissolution of animal and vegetable bodies.
" The air is very instrumental in the production and growth of animals and vegetables, both by invigorating their several juices while in an elastick active state, and also by greatly contributing in a fix'd state to the union and firm connection of several constituent parts of those bodies, viz. their water, salt, sulphur, and earth. This band of union, in conjunction with the external air, is also a very powerful agent in the dissolution and corruption of the same bodies ; for it makes one in every fermenting mixture ; the action and re-action of the aerial and sulphureous particles is, in many fermenting mixtures, so great as to excite a burning heat, and in others a sudden flame ; and it is, we see, by the like action and re-action of the same principles, in fuel and the ambient air, that common culinary fires are produced and maintained.
" Tho' the force of its elasticity is so great as to be able to bear a prodigious pressure, without losing that elasticity, yet we have, from the foregoing Experiments, evident proof that its elasticity is easily and in great abundance destroyed ; and is thereby reduced to a fixt state by the strong attraction of the acid sulphureous particles which arise either from fire or from fermentation ; and therefore elasticity is not an essential immutable property of air-particles ; but they are, we see, easily changed from an elastick to a fixt state, by the strong attraction of the acid, sulphureous, and saline particles which abound in air. Whence it is reasonable to conclude that our atmosphere is a Chaos, consisting not only of elastick, but also of unelastick air-particles, which in plenty float in it, as well as the sulphureous saline, watry, and earthy particles, which are no ways capable of being thrown off into a permanently elastick state, like those particles which constitute true permanent air. Since, then, air is found so manifestly to abound in almost all natural bodies ; since we find it so operative and active a principle in every chymical operation ; since its constituent parts are of so durable a nature, that the most violent action of fire or fermentation cannot induce such an alteration of its texture as thereby to disqualify it from resuming, either by the means of fire or fermentation, its former elastick state: unless in the case of vitrification, when, with the vegetable Salt and Nitre in which it is incorporated, it may, perhaps, some of it, with other chymical principles, be immutably fixt, since then this is the case, may we not with good reason adopt this now fixt, now volatile Proteus among the chymical principles, and that a very active one, as well as acid sulphur ; notwithstanding it has hitherto been overlooked and rejected by chymists, as no way entitled to that denomination ? "
This quotation shows us how little Mayow's shrewd reasoning and well-devised experiments had impressed the thinkers of his age. While Hales quotes frequently from Boyle's and Newton's works, his reference to Mayow is meagre ; nor does he adopt any one of Mayow's conclusions. One would have thought that, having prepared so many gases by means of apparatus well adapted to their purpose, and having observed that certain substances introduced into air produced contraction, he would have drawn the conclusion that such " airs " were essentially different kinds of matter. But the " Proteus " was too much for him ; and he left the subject practically in the same state of " Chaos" in which he found it.





Comments