As we exit the worst of the COVID-19 pandemic, the health care community must consider how to apply lessons learned over the past year to improve quality of care and patient outcomes across the health care spectrum. One critical advancement made during COVID was the growing reliance on the life-saving power of Real-World Evidence (RWE) – enabled by the widespread adoption of electronic health records (EHRs), the availability of diverse data sets, and advancements in analytics – all of which allowed researchers to gather data from everyday healthcare settings and collect real-time insights into disease epidemiology and treatment effectiveness. RWE was essential in understanding and fighting COVID-19, and now we must urgently expand their use, particularly in fields setback by the pandemic, such as oncology.
Throughout the past year, researchers have relied on RWE to examine existing drugs to treat COVID-19 and guide health care decision-making — from modeling how travel restrictions affect global spread, to evaluating the effectiveness of the COVID-19 vaccines against new variants, to determining that the benefits of vaccination far outweigh the risks.
If RWE can be effectively adopted in oncology, it has the potential to mitigate some of the calamitous effects of the pandemic on traditional clinical cancer research and deliver transformational insights about patient care, treatment pathways, and drug effectiveness.
Here are three reasons why:
First, RWE studies represent real-world patient populations in real-life settings. Traditionally, only a small percentage of cancer patients qualify for randomized controlled clinical trials due to strict selection criteria. Unsurprisingly, this narrow trial population often does not reflect the real-world patient population, which tends to be older, less healthy, and more diverse. RWE studies, on the other hand, can help researchers better understand how diseases and their treatments affect underrepresented groups. Researchers already rely on RWE to support research in rare patient cohorts – such as older patients and minority groups. And, RWE address challenges with enrolling sufficient numbers of participants in traditional clinical trials. Now it’s even easier to attract real-world patients because RWE studies don’t require that they do much more than grant access to their data, eliminating risks posed by going to a hospital or doctor’s office for tests.
Second, RWE can elevate patient-centricity. In traditional clinical trials, patient involvement is rarely considered. Today’s emerging RWE studies, and innovative trial design approaches, have “reset” the perception of study participants as valued partners. For example, the GRECO-breast cancer study – which is currently recruiting – will incorporate the participants’ experience, from protocol development to research completion. The study, which will examine EHRs and radiological data from participants to evaluate the role of our liquid biopsy test in determining treatments and outcomes, will also incorporate the patient’s self-reported experiences along the way. Patient privacy is protected by fully anonymizing data, and every possible enrollment burden has been removed, resulting in a more diverse and representative population. This patient-centric approach will ultimately collect more longitudinal information, reducing challenges related to data silos or lack of interoperability. 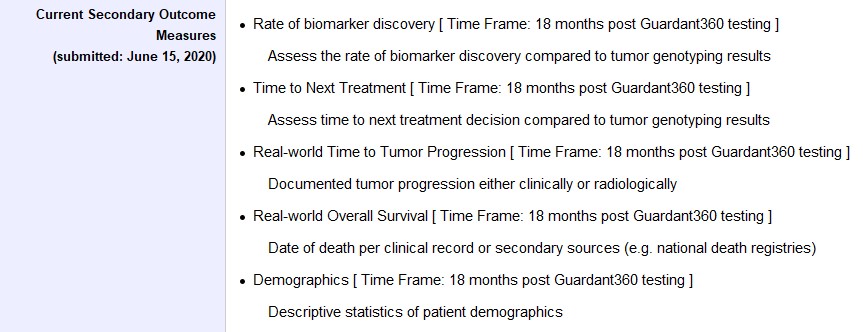
Finally, RWE studies identify significant findings more quickly. When the pandemic hit, many theorized that cancer patients may be more susceptible to severe COVID-infections due to weakened immune systems. A traditional study to evaluate this hypothesis could take years; but a RWEstudy conducted by the FDA that reviewed the health records of 212,000 cancer patients confirmed – just four months after the pandemic began in the U.S. – that cancer patients with COVID-19 were, in fact, more likely to be hospitalized, ventilated, and die. As a result, cancer patients were encouraged to exercise greater caution and were included on the CDC’s list of people at higher risk. Looking ahead RWE studies are able to be completed faster because much of the data – including EHRs, claims and billing activities, and product or disease registries – already exist. And patient-generated data from wearables, mobile phones and other technologies can also be expected to be incorporated over time.
The use of RWE studies during the pandemic enabled researchers, doctors, and public health officials to adapt quickly to an ever-changing environment. Now we must adopt these lessons and the use of RWE to mitigate the devastating effects the COVID-19 pandemic has had on the cancer ecosystem. Over the past year, diagnostic screenings have been postponed, non-urgent surgeries rescheduled, and clinical trials have been delayed or cancelled. But the wider adoption of RWE studies across cancer research can be a positive development that emerges from this crisis, mitigating the devastating effects COVID-19 has had on cancer research, and changing the oncology research landscape for the better. We must come together as an industry to seize this life-saving opportunity.





Comments