Nano-structures become very complex; for example, catalysis is routinely achieved bi-metallically,[2] with bi-metallic wires,[3] dendrites [4] etc., where the compounds can have two separated types of metallic nano-particles or alloyed combinations. We already witnessed the extension to tri-metallic compounds with the nano-particles themselves being also multi-metallic becoming usual. [5,6,7] The complexity is increased by the use of nano-structured matrixes like silica [8] or polymers [9] and carbon nano-tubes [10] or spheres (see Fig. 1), [11] which do far more than only passively dispersing the “active material”, thereby avoiding its agglomeration. Even without direct particle-matrix interactions, every added degree of freedom allows to tune properties, say by particle distributions [12] and location control. [13] Such allows further optimization.
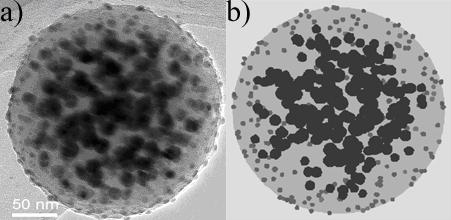
Fig. 1 A porous amorphous carbon micro sphere with Ag particles inside and Au particles on the outside, thus separating the different metals, (a) its transmission electron microscopy (TEM) image and (b) a computer simulation that helped analyzing the structure quantitatively, revealing the very low density of the carbon.
We discuss structurally complex as well as, for example, regarding electron distribution complex environments. They may create particular absorbance sites for molecules while also supplying surplus electrons to an intermediate reaction, but such details are almost beside the point. Faced with complex reactions and no way to either fully experimentally control nor to theoretically predict overall performance as dependent on temperature etc., it is the complexity as such that creates an abundance of sufficiently versatile niches which allow reactions to proceed.
Important is the versatility of tasks that can be provided. For example, the addition of Pd or Pt adds hydrogen absorbance, but with no way to anticipate how to best put them into the whole structure. There is an embarrassment of riches in the ways one can add Pd into a modern compound such as a porous carbon sphere seeded with Ag inside and Au nanoparticles outside (Fig. 1).
Shall we put a certain size and shape of the new metal’s crystals just above the carbon surface but not contacting the Ag just below the surface [14] or should we try putting the new metal into already present ones such as in Fig. 2, increasing the degree of alloying? The complexity attainable by adjusting alloying* alone is very large.
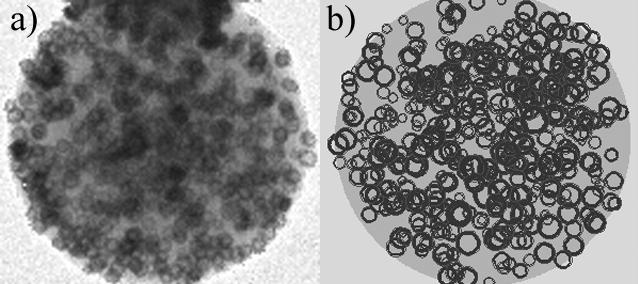
Fig. 2 A carbon micro sphere with Ag nano-particles partially hollowed out and replaced by Pd on the outside of each nano-particle, leading to a complex structure where the different metals are close together without constituting an alloy, (a) the TEM image and (b) a computer simulation of the replacement process which revealed that the TEM is very misleading toward the percentage of the Ag being replaced by Pd. The impression of very large cavities arises even at small replacement ratios (here only 1/3 of the original nano-particles’ volume has been removed).
The problem is not the size of parameter spaces but dimensionality. Even a very large one dimensional parameter space can be well investigated by simply scanning through from one end to the other.
Complex structures have many degrees of freedom and therefore parameter spaces with many dimensions. Metal-nano-particle-dielectric micro-matrix compounds have tens of parameters that all influence catalysis, starting with the porosity and overall size of the matrix particles, which influences also their own mutual aggregation.
We can neither efficiently survey nor optimize in such dimensionally large spaces, for example find the optimum structure that catalyses a specific reaction. Surveying such parameter spaces needs multi-sample preparation and analysis, but even then it is prohibitively resource expensive by brute force.
Efficient survey and optimization strategies are needed. These are also difficult because of the strong interdependence of parameters. For instance, every added metal supplies not only another total amount of metal, another size distribution of the new particles, and their location relative to the locations of the particles of the other metals in the matrix. Also many of the old parameters are changed, such as the perhaps previously optimized porosity. The additional adjustable parameters (large-dimensionality) are strongly coupled (interdependence), for example the shapes of the partially replaced metal particles all change with the added metal because of the partial replacement of their atoms.
Predictability is lost and understanding often questionable: Does the addition of a metal via a galvanic replacement reaction add new ‘synergistic’ metal/metal interfaces or did the reaction merely make the original metal more porous, and how to find out?
This article continues “Adapting As Nano Approaches Biological Complexity: Witnessing Human-AI Integration Critically” where the status of all this as suppressed information has been discussed. I allow myself to actually publish the most interesting and critical parts here (slightly edited). If citing, please cite nevertheless [1] anyway in order to support the author.
Next week's post will continue with the title
"Magic of Complexity with Catalysts Social or Metallic," then
"Emergent Parameters and High Throughput Screening" before finally getting to
"Critique of Nanotech: The most dangerous Science least carefully done"
and perhaps I also add
"A flexible, evolving approach to computing."
------------------
*Alloying: We define atomic alloying as the merging of the different metals’ XRD refraction peaks. If the different metals are distributed into pure grains (nano-alloying), the single peak broadens and, depending on grain sizes and distribution, the pure metal’s shifted refraction peaks appear either alone or with mixed metals’ signals still between them. Once the pure grains are too large, XRD cannot distinguish degrees of alloying. TEM shows whether domains of different metals intermingle or whether the different metals’ grains accumulated into large pure and perhaps even isolated domains (no alloying).
------------------
[1] S. Vongehr et al., Adapting Nanotech Research as Nano-Micro Hybrids Approach Biological Complexity. Journal of Materials Science&Technology 32(5), 387-401 (2016)
doi: 10.1016/j.jmst.2016.01.003 www.jmst.org/EN/abstract/abstract24512.shtml.
[2] C. K. Shi and P. Zhang, Effect of a second metal (Y, K, Ca, Mn or Cu) addition on the carbon dioxide reforming of methane over nanostructured palladium catalysts. Appl. Catal. B Environ., 2012, 115, 190-200.
[3] C. Z. Zhu, S. J. Guo and S. J. Dong, PdM (M = Pt, Au) Bimetallic Alloy Nanowires with Enhanced Electrocatalytic Activity for Electro-oxidation of Small Molecules. Adv. Mater., 2012, 24, 2326–2331.
[4] J. F. Huang, S. Vongehr, S. C. Tang, H. M. Lu, J. C. Shen and X. K. Meng, Ag Dendrite-Based Au/Ag Bimetallic Nanostructures with Strongly Enhanced Catalytic Activity. Langmuir, 2009, 25, 11890-11896.
[5] C. Z. Zhu, S. J. Guo, and S. J. Dong, Facile synthesis of trimetallic AuPtPd alloy nanowires and their catalysis for ethanol electrooxidation. J. Mat. Chem., 2012, 22, 14851-14855.
[6] C. K. Poh, Z. Q. Tian, J. J. Gao, Z. L. Liu, J. Y. Lin, Y. P. Feng and F. B. Su, Nanostructured trimetallic Pt/FeRuC, Pt/NiRuC, and Pt/CoRuC catalysts for methanol electrooxidation. J. Mat. Chem., 2012, 22, 13643-13652.
[7] C. J. Zhong, J. Luo, B. Fang, B. N. Wanjala, P. N. Njoki, R. Loukrakpam and J. Yin, Nanostructured catalysts in fuel cells. Nanotechnology, 2010, 21, # 062001, pp 1-20.
[8] C. W. Yen, M. L. Lin, A. Q. Wang, S. A. Chen, J. M. Chen and C. Y. Mou, CO Oxidation Catalyzed by Au-Ag Bimetallic Nanoparticles Supported in Mesoporous Silica. J. Phys. Chem. C, 2009, 113, 17831–17839.
[9] S. F. Zhang, W. Wu, X. H. Xiao, J. Zhou, J. X. Xu, F. Ren and C. Z. Jiang, Polymer-Supported Bimetallic Ag@AgAu Nanocomposites: Synthesis and Catalytic Properties. Chem. An Asian J., 2012, 7, 1781-1788.
[10] J. M. Sieben and M. M. E. Duarte, Methanol, ethanol and ethylene glycol electro-oxidation at Pt and Pt-Ru catalysts electrodeposited over oxidized carbon nanotubes. Int. J. Hydr. Energy, 2012, 37, 9941-9947.
[11] S. C. Tang, S. Vongehr and X. K. Meng, Controllable incorporation of Ag and Ag-Au nanoparticles in carbon spheres for tunable optical and catalytic properties. J. Mater. Chem., 2010, 20, 5436-5445.
[12] S. C. Tang, S. Vongehr, Z. Zheng, H. J. Liu and X. K. Meng, Silver Doping Mediated Route to Bimetallically Doped Carbon Spheres with Controllable Nanoparticle Distributions. J. Phys. Chem. C, 2010, 114, 18338-18346.
[13] S. C. Tang, S. Vongehr, G. R. He, L. Chen and X. K. Meng, Highly Catalytic Spherical Carbon Nanocomposites allowing Tunable Activity via controllable Au-Pd Doping. J. Coll.&Interf. Sci., 2012, 375, 125-133.
[14] S. C. Tang, S. Vongehr and X. K. Meng, Layered Spherical Carbon Composites with Nanoparticles of Different Metals Grown Simultaneously Inside and Outside. Nanotech., 2012, 23, 095603-095614.





Comments